Abstract
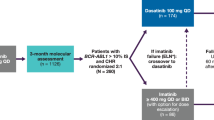
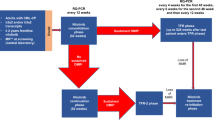
Dasatinib, a potent inhibitor of BCR–ABL in vitro, is effective for patients with chronic myelogenous leukemia (CML) resistant or intolerant to imatinib. To provide a more definitive assessment of dasatinib in chronic-phase (CP)-CML, we report extended follow-up of a phase II trial, presenting data for the entire patient cohort (N=387). Dasatinib (70 mg) twice daily was administered to patients with imatinib-resistant or -intolerant CP-CML. With median follow-up of 15.2 months (treatment duration, <1–18.4 months), a complete hematologic response was attained or maintained in 91% of patients. A major cytogenetic response (MCyR) was attained or maintained by 59% (52% imatinib resistant and 80% imatinib intolerant); this was complete in 49% of patients (40% imatinib resistant and 75% imatinib intolerant). Of 230 patients achieving an MCyR, 7 experienced disease progression. Fifteen-month progression-free survival was 90% while overall survival was 96%. Grade 3/4 thrombocytopenia and neutropenia were reported in 48 and 49% of patients, respectively. Non-hematologic toxicity (any grade) consisted primarily of diarrhea (37%), headache (32%), fatigue (31%), dyspnea (30%) and pleural effusion (27%). Pleural effusions were classified as grade 3 in 6% of reported events, with no incidence of grade 4. Dasatinib is associated with high response rates in patients with imatinib-resistant or -intolerant CP-CML.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Faderl S, Talpaz M, Estrov Z, O’Brien S, Kurzrock R, Kantarjian HM . The biology of chronic myeloid leukemia. N Engl J Med 1999; 341: 164–172.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood 2006; 108: 1809–1820.
Hochhaus A, Hughes T . Clinical resistance to imatinib: mechanisms and implications. Hematol Oncol Clin North Am 2004; 18: 641–656.
Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J et al. Favorable long-term follow-up results over six years for response, survival and safety with imatinib mesylate therapy in chronic phase chronic myeloid leukemia post failure of interferon-alpha treatment. Blood 2008; 111: 1039–1043.
Druker BJ, Guilhot F, O’Brien S, Gathmann I, Kantarjian H, Gattermann N et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006; 355: 2408–2417.
O’Hare T, Walters DK, Stoffregen EP, Jia T, Manley PW, Mestan J et al. In vitro activity of Bcr–Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res 2005; 65: 4500–4505.
Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem 2004; 47: 6658–6661.
Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic phase chronic myeloid leukemia after failure of imatinib therapy. Blood 2007; 109: 2303–2309.
Clopper CJ, Pearson ES . The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934; 26: 404–413.
Brookmyer R, Crowley JJ . A confidence interval for the median survival time. Biometrics 1982; 38: 29–41.
Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 2006; 354: 2542–2551.
Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL . Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 2004; 305: 399–401.
Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN et al. Clinical resistance to STI-571 cancer therapy caused by BCR–ABL gene mutation or amplification. Science 2001; 293: 876–880.
Hochhaus A, Kreil S, Corbin AS, La Rosée P, Müller MC, Lahaye T et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002; 16: 2190–2196.
Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R et al. BCR–ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood 2003; 101: 690–698.
Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlägel U, von Bonin M et al. P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 2004; 18: 401–408.
Thomas J, Wang L, Clark RE, Pirmohamed M . Active transport of imatinib into and out of cells: implications for drug resistance. Blood 2004; 104: 3739–3745.
Dai Y, Rahmani M, Corey SJ, Dent P, Grant S . A Bcr/Abl-independent, Lyn-dependent form of imatinib mesylate (STI-571) resistance is associated with altered expression of Bcl-2. J Biol Chem 2004; 279: 34227–34239.
Quintas-Cardama A, Kantarjian HM, O’Brien S, Borthakur G, Bruzzi J, Munden R et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol 2007; 25: 3908–3914.
SPRYCEL (package insert). Dasatinib Prescribing Information. Bristol–Myers Squibb: New York, NY. November 2007.
Acknowledgements
In addition to the authors, the following primary investigators also participated in this trial: Australia: C Arthur, S Branford (molecular analyses), A Grigg, J Seymour and K Taylor; Austria: P Valent; Belgium: A Bosly; Canada: D Forrest, C Gambacorti-Passerini and P Laneuville; Denmark: JL Nielsen; Finland: K Porkka; France: J-L Harrousseau, F Maloisel, M Michallet, J Reiffers and P Rousselot; Germany: C Bokemeyer, P Erben (molecular analyses) and T Fischer; Ireland: E Conneally and M O’Dwyer; Israel: A Nagler; Italy: E Abruzzese, F Castagnetti, F Ferrara, G Lambertenghi, V Liso, G Rosti, B Rotoli and G Saglio; Korea: D-W Kim; Netherlands: J Cornelissen and AVMB Schattenberg; Peru: J Navarro; Singapore: YT Goh; Spain: J Odriozola and JL Steegmann; Sweden: M Ekblom, B Markevarn, B Simonsson and L Stenke; Switzerland: A Gratwohl; UK: T Holyoake; USA: K Bhalla, S Bilgrami, B Cheson, R Collins, S Coutre, J Dipersio, S Durham, L Fehrenbacher, JK Giguere, FA Greco, HJ Khoury, M Lilly, J Lister, S Luger, A Maniam, J McGuirk, E Merriam, MS Murali, J Radich (molecular analyses), A Rapoport, CE Rivera, C Schiffer, R Strair and M Talpaz. Professional writing and editorial assistance, funded by Bristol–Myers Squibb, was provided by Josh Collis and Andrew Richardson. The funding for this trial was provided by Bristol–Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Additional information
This trial (trial no. CA180-013) is registered at http://www.clinicaltrials.gov.
Rights and permissions
About this article
Cite this article
Hochhaus, A., Baccarani, M., Deininger, M. et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 22, 1200–1206 (2008). https://doi.org/10.1038/leu.2008.84
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2008.84
Keywords
This article is cited by
-
Which Second-Line Tyrosine Kinase Inhibitor(s) for Chronic Myeloid Leukemia?
Current Treatment Options in Oncology (2023)
-
The Evolving Landscape of Frontline Therapy in Chronic Phase Chronic Myeloid Leukemia (CML)
Current Hematologic Malignancy Reports (2021)
-
Elucidation of protein interactions necessary for the maintenance of the BCR–ABL signaling complex
Cellular and Molecular Life Sciences (2020)
-
Synthesis and biological evaluation of phenyl-amino-pyrimidine and indole/oxindole conjugates as potential BCR-ABL inhibitors
Medicinal Chemistry Research (2019)
-
Is There a Role for Dose Modification of TKI Therapy in CML?
Current Hematologic Malignancy Reports (2019)