Abstract
Scleroderma is a fibrosis-related disorder characterized by cutaneous and internal organ fibrosis, and excessive collagen deposition in extracellular matrix (ECM) is a major cause of fibrosis. Transforming growth factor-β (TGF-β)/SMAD signaling has a central role in the pathogenesis of fibrosis by inducing abnormal collagen accumulation in ECM, and latent TGF-β-binding protein 4 (LTBP-4) affects the secretion of latent TGF-β to ECM. A previous study indicated that bleomycin (BLM) treatment increased LTBP-4 expression in lung fibroblasts of Thy-1 knockout mice with lung fibrosis, and LTBP-4 further promoted TGF-β bioavailability as well as SMAD3 phosphorylation. However, the expression and function of LTBP-4 in human scleroderma remain unclear. We aimed to investigate the potential role of LTBP-4 in scleroderma through clinical, in vivo and in vitro studies. LTBP-4 and TGF-β expressions were significantly upregulated in systemic scleroderma (SSc) patients’ plasma compared with normal controls (LTBP-4, 1,215±100.2 vs 542.8±41.7 ng/ml, P<0.0001; TGF-β, 1.5±0.2 vs 0.7±0.1 ng/ml, P=0.0031), while no significant difference was found between localized scleroderma (LSc) and normal controls. The plasma concentrations of LTBP-4 and TGF-β were even higher in SSc patients with lung fibrosis (LTBP-4, 1462± 137.3 vs 892.8±113.4 ng/ml, P=0.0037; TGF-β, 2.0±0.4 vs 0.9±0.2 ng/ml, P=0.0212) and esophagus involvement (1390±134.4 vs 940.7±127.0 ng/ml, P=0.0269; TGF-β, 1.9±0.3 vs 0.9±0.2 ng/ml, P=0.0426). The area under receiver operating characteristics (ROC) curve of LTBP-4 was 0.86. Immunohistochemistry measurement also demonstrated a higher LTBP-4 expression in sclerotic skin tissue of LSc and SSc compared with normal controls. More positive fibroblasts were also found in BLM-induced scleroderma mouse model than the saline-treated group. In in vitro studies, knockdown of LTBP-4 in SSc skin fibroblasts prominently reduced downstream COL1A1, COL1A2, and COL3A1 mRNA level by 84%, 82%, and 43%, respectively, and other fibrosis-related genes’ expression were also decreased. Furthermore, extracellular TGF-β level and the SMAD2/3 phosphorylation were inhibited through LTBP-4 knockdown treatment, suggesting that the knockdown of LTBP-4 reduced the collagen expression through TGF-β/SMAD signaling pathway. Taken together, these data suggest that LTBP-4 affects fibrotic process in scleroderma, and the high expression of LTBP-4 in SSc plasma may serve as a clinical biomarker in diagnosing this disease. In addition, this study also lays the theoretical foundation for targeting LTBP-4 as treatment of scleroderma.
Similar content being viewed by others
Main
Scleroderma is a typical connective tissue disease featured by collagen overproduction. Two categories of scleroderma have been described: systemic scleroderma (SSc) and localized scleroderma (LSc). The former is characterized by cutaneous sclerosis and visceral involvement (especially the esophagus, lung, and vascular system) that is associated with a worse prognosis and a high mortality rate; the latter is confined to the skin and/or underlying tissues, presenting benign and self-limited fibrosis.1 Although LSc and SSc have a number of different disease presentations, both share the common clinical hallmark of skin fibrosis characterized by excessive transforming growth factor-β (TGF-β) activity and extracellular matrix (ECM) production.2, 3 However, underlying mechanisms of this phenomenon are still unclear.4
Several pro-fibrotic cytokines have been implicated in fibrotic process of scleroderma, including TGF-β, connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 (PAI-1), fibronectin 1 (FN-1), and others.5, 6 Among these cytokines, TGF-β is considered the principal mediator of skin fibrosis in the pathogenesis of scleroderma. There are two known structurally distinct latent complexes of TGF-β: small latent TGF-β complex (SLC), which consists of a TGF-β domain, and the latency-associated peptide (LAP). SLC further forms the large latent complex (LLC) with the latent TGF-β-binding proteins (LTBPs). After secretion and deposition of LLC in the ECM, the mature TGF-β dimer is activated by its dissociation from LAP and LTBP, which can be accomplished by proteolysis.7 Then, TGF-β binds to its receptors and phosphorylates SMAD2 and SMAD3 proteins. The phosphorylated SMAD proteins form a complex with SMAD4 on SMAD-binding element (SBE) in the nucleus and regulates transcriptional responses of diverse genes mentioned above.8, 9
LTBPs belong to the family of fibrillin/LTBP glycoproteins. Like the fibrillins, LTBPs are structural components of the ECM microfibrils, which are involved in the deposition of TGF-β in the ECM.10 In addition, LTBPs also facilitate latent TGF-β secretion and regulate latent TGF-β activation.11 Currently, four different LTBPs have been cloned from mammals (LTBP1–4). It has been shown that dysregulated expression of LTBP isoforms are related to the onset of various carcinomas.12, 13, 14, 15, 16, 17 In addition, LTBP-4 is also associated with fibrosis-related disease, which is unique within those isoforms. A recent study showed that LTBP-4 exerted distinct functions by causing autosomal recessive cutis laxa type 1C in mice.18 For lung fibrosis, it is known that LTBP-4 activates TGF-β bioavailability as well as SMAD3 phosphorylation in lung fibroblasts of bleomycin (BLM)-treated Thy-1 knockout mice.19
Despite these previous studies, the role for LTBP-4 in the process of dermal fibrosis in scleroderma is currently unknown. Our research team previously found a markedly higher level of LTBP-4 in BLM-treated mice through gene expression chips. We hypothesized that LTBP-4 is involved in the mechanism of scleroderma fibrosis, which needs to be verified by further investigation. The intention of this study was to perform a quantitative analysis of LTBP-4 expression in skin tissue and plasma of scleroderma patients. We also analyzed whether the inhibition of LTBP-4 in dermal fibroblasts attenuated collagen gene expression and further modulated the activation of TGF-β/SMAD signaling.
Materials and methods
Patients
A total of 46 Chinese patients with SSc and 43 patients with LSc who fulfilled the criteria for diagnosis were enrolled from Shanghai Traditional Chinese Medicine-Integrated Hospital and Huashan Hospital. The medical records were reviewed retrospectively, including medical histories, physical examinations, and laboratory tests. Most of the scleroderma patients in our studies have a medication history of oral glucocorticoid (anti-inflammation and immunosuppression) and snow glycosides tablets (antifibrosis). Forty-six age- and gender-matched healthy Chinese individuals were enrolled as normal controls. The characteristics of scleroderma patients and healthy individuals are listed in Table 1. Also, the extent of organ involvement was reviewed in the SSc group and is shown in Table 1. Evaluation of organ involvements was performed according to the criteria outlined by Sato et al:20 pulmonary fibrosis=bibasilar fibrosis on chest radiography and high-resolution CT, esophagus involvement=hypomotility shown by barium radiography, arthralgia=inflammatory polyarthralgia, heart involvement=pericarditis, congestive heart failure, or arrhythmias requiring treatment. This study was approved by the Institutional Review Board of Huashan Hospital and School of Life Sciences, Fudan University, and informed consent was obtained from all participants for the sample collection and subsequent analysis.
Establishment of Skin Fibrosis Mouse Model
Specific pathogen-free, female C57BL/6J mice (7-week old; Sino-British Sippr/BK Lab Animal, Shanghai, China) were used to generate the animal model at the Animal Centre of the State Key Laboratory of Genetic Engineering, School of Life Sciences, Fudan University. The skin fibrosis mouse model was performed using 200 μg/ml BLM (Nippon Kayaku, Tokyo, Japan) dissolved in saline (Sa). Injection with BLM was administered (100 μl) subcutaneously into the same site of the shaved upper back daily. Filter-sterilized Sa was administrated in control group with the same dosage and method. All animals were killed at the end of 3 weeks by anesthesia and necropsies were carried out using standard protocols. The animals used in experiment were approved by the School of Life Sciences, Fudan University, China, and the animal protocols were performed in accordance with the approved guidelines.21
Immunohistochemical Staining
Human skin tissues were obtained from 20 scleroderma patients (10 LSc and 10 SSc) and 10 healthy individuals. The characteristics of scleroderma patients and healthy individuals involved are listed in Table 2. Mouse tissues came from five BLM-induced mice and five Sa-treated animals as described above. Tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Hematoxylin/eosin (H&E) and Masson’s trichrome staining were used to detect tissue structure. Nikon Eclipse 80i microscope (Nikon, Badhoevedorp, the Netherlands) was used to measure dermal thickness. The maximal distance between the epidermal–dermal junction and the dermal–subcutaneous fat junction in all skin sections described above was analyzed according to the method mentioned by Yoshizaki et al.22 The evaluation was performed by two independent examiners. For human and mouse immunohistochemistry (IHC), LTBP-4 (1:50; Santa Cruz Biotechnology, TX, USA), S100A4 (1:500; Cell Signaling Technology, MA, USA) and anti-α-smooth muscle actin (α-SMA; 1:500; Millipore, MA, USA) were used as primary antibody. Goat anti-mouse IgG and goat anti-rabbit IgG were used as secondary antibodies. The tissue sections were then examined under the microscope.
LTBP-4 and TGF-β Quantification ELISA
Blood samples were collected in EDTA-containing tubes and plasma was isolated within 1 h from the blood and stored at −80 °C until the analysis. LTBP-4 level was quantified using a LTBP-4 ELISA kit (EIAab, Wuhan, China). All samples were assayed according to the manufacturer’s instructions. The 96-well microplate wells were coated with a monoclonal antibody specific for LTBP-4. Eight different concentrations of the standard material and 100 μl of each patient’s plasma were transferred into each well of the ELISA plate. After incubation, the unbound conjugate was washed off and a substrate solution was added to each well. The optical density of each well was determined using a microplate reader at 450 nm. Plasma TGF-β and total TGF-β levels in supernatant of human skin fibroblasts (HSFs) were determined using the Human TGF-β Quantikine ELISA kit (R&D Systems, MN, USA) following acidification of the sample to activate latent TGF-β according to the manufacturer’s instructions.
Cell Culture
HSFs were obtained from three limited cutaneous SSc patients’ biopsy specimens. The average age was 42.4±1.7 years (mean±s.e.m.). HSFs were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen, CA, USA) supplemented with 10% fetal bovine serum (FBS) at 37 °C and 5% CO2. Cells were cultured until 80–90% confluency and detached from tissue culture dishes with 0.25% trypsin. Cell purity was assessed by flow cytometry analysis. As shown in Supplementary Figure 1, HSF isolated form SSc skin demonstrated a high positivity (99.5%) for S100A4 expression, indicating that the cultured cells were fibroblasts.
siRNA Knockdown of Human LTBP-4
HSF cells were cultured overnight on 12-well plates at a density of 1 × 105 cells per well in the medium containing 10% FBS. Human LTBP-4 siRNA and negative control siRNA (Origene, MD, USA) were transfected into cells at a final concentration of 10 nmol/l for each well using Lipofectamine RNAiMAX reagent (Invitrogen; prepared according to the manufacturer’s directions). Then, cells were incubated for another 48 h for RNA and protein collected. The supernatants of HSF cells were collected for quantification of TGF-β.
RNA Isolation, Reverse Transcription, and Real-Time PCR
Trizol reagent (Invitrogen) was used for the isolation of RNA according to the manufacturer’s instructions. RNA concentrations and purities were determined spectrophotometrically. Reverse transcription was done using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA) according to the manufacturer’s instructions. For real-time PCR (RT-PCR) analysis, a SYBR Green I PCR Kit (TaKaRa, Shiga, Japan) was used according to the manufacturer’s instructions. Reactions were performed in a 384-well format using 5 μl total volume per well. The relative expression of the LTBP-4 in the RT-PCR was calculated according to the ΔΔCt method using an internal reference sample as calibrator.23
Western Blot
The cell lysates extracted from the cultured cells were used for immunoblotting analysis. Proteins were extracted from cells, and protein concentration was determined with a BCA Assay Kit (Vazyme, Nanjing, China). Equal amounts of protein were loaded onto 10% SDS-PAGE gel and transferred to PVDF membranes (Millipore). The membranes were blocked with 5% bovine serum albumin at room temperature for 1 h. Then, the membranes were incubated overnight at 4 °C with anti-LTBP-4 antibody (1:100; Santa Cruz Biotechnology), goat anti-Collagen type I antibody (1:500; Millipore), anti-SMAD2/3 antibody (1:1000; Cell Signaling Technology), anti-pSMAD2/3 antibody (1:1000; Cell Signaling Technology), and internal control GAPDH antibody (Cell Signaling Technology). After three washes with TBST for 30 min, the PVDF membranes were incubated with appropriate secondary antibodies for 1 h. Detection was performed using an enhanced chemiluminescence system, and the intensity of bands was quantified using the Image-QuantTL software (General Electric Company, CT, USA).
Luciferase Reporter Gene Assay
The cells were cultured in DMEM supplemented with 10% FBS for 24 h in 12-well plates before transfection. Specific human LTBP-4 siRNA and negative control were transfected into cells as described previously. Meanwhile, 500 ng pGL4-SBE-Lucvector (Promega, WI, USA) that contains a SMAD-binding site upstream of luciferase gene, and 10 ng control vector, pRL-SV40 (Clontech, CA, USA), were co-transfected using Lipofectamine 2000 (Thermo, MA, USA). After 24 h of transfection, luciferase reporter gene assay was performed with a dual luciferase assay system and the manual of the manufacturer (Promega).
Statistical Analysis
An independent two group t-test or one-way ANOVA test were used to determine the significance of differences between groups. Pearson correlation coefficients were calculated to explore associations between plasma LTBP-4 and TGF-β levels. P<0.05 was considered statistically significant. The statistical analyses were performed with the SPSS 16.0 software.
Results
LTBP-4 Expression was Enhanced in BLM-Induced Scleroderma Mouse Model
Our previous gene expression chip study had revealed an increased LTBP-4 expression in a scleroderma mouse model which has similar histological and molecular hallmarks compared with SSc patients.24, 25 To further confirm the potential role of LTPB-4 in scleroderma, the LTBP-4 expression level was examined in the skin and fibroblasts of BLM-induced mouse model. H&E and Masson’s staining revealed that BLM mice had a thicker dermis (Sa vs BLM: 0.8±0.1 vs 1.8±0.2 mm, P<0.05) and more collagen contents than Sa-treated ones (Figure 1a and b). In IHC staining, more positive cells expressing α-SMA were observed in the skin of BLM mice compared with normal tissues. Similarly, tissues from BLM mice also had more cells positive for LTBP-4 staining. Large amounts of LTBP-4 or α-SMA-positive cells were also involved in gland tissues (Figure 1a). In addition, the transcription levels of Col1a1, Col1a2, Col3a1, Ctgf, and PAI-1 were detected in the mouse model. As a result, all the collagen-related genes mentioned above showed an apparent increase in the BLM group compared with control group, which led to a further increase of Ltbp-4 mRNA level in the BLM group (Figure 1c).
Expression of α-SMA and LTBP-4 was increased in the skin lesions of BLM-treated mice. (a) H&E, Masson’s staining, and IHC for α-SMA, LTBP-4 in saline-treated mice, and BLM mice. For H&E and Masson’s staining, the original magnification is × 200 and the scale bar is 100 μm. For α-SMA and LTBP-4 IHC, the original magnification is × 400 and the scale bar is 100 μm. Black arrow indicates α-SMA-positive staining and LTBP-4-positive staining. (b) Dermal thickness was calculated at × 200 microscopic magnification by measuring the distance between the dermal–epidermal junction and the derma–subcutaneous fat junction (μm; as indicated by H&E staining) in five randomly selected fields for each skin section. (c) Expression of Ltbp-4 and other ECM-related gene level in mouse skin were increased in the BLM group compared with the saline-treated group; the mRNA levels were calculated using a relative ratio to Gapdh. Bars show the mean±s.e.m. results from five mice in each group. **P<0.01, *P<0.05. H&E, hematoxylin/eosin; IHC, immunohistochemistry; LTBP-4, latent TGF-β-binding protein 4; α-SMA, α-smooth muscle actin.
LTBP-4 Expression was Enhanced in The Skin of Scleroderma Patients
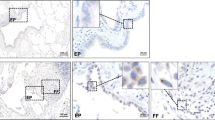
Skin tissue sections were collected from healthy individuals, and LSc and SSc patients. H&E staining presented an apparent increase in dermis thickness in the LSc and SSc group (Control: 1.6±0.1 mm, LSc: 7.4±0.4 mm, SSc: 8.1±0.2 mm, P<0.0001). In parallel, more collagen contents were observed in dermis from Masson’s staining in the LSc and SSc group (Figure 2a and b). Further IHC was performed on sections stained with the primary antibodies for LTBP-4 and α-SMA. As shown in Figure 2c, the number of positive cells for α-SMA and LTBP-4 were elevated in LSc and SSc patients compared with the Sa-treated group. Similar to BLM mice, abundant positive cells were also present in glands. Besides, LTBP-4-positive cells and S100A4-positive cells were also observed at the same regions of the skin tissue sections, indicating that the cells expressing LTBP-4 were fibroblasts (Supplementary Figure 2).
Expression of α-SMA and LTBP-4 was increased in the skin lesions of scleroderma patients. (a) H&E and Masson’s staining for the human skin tissues of normal control, LSc, and SSc. Original magnifications: × 40, scale bars: 1000 μm. (b) Dermal thickness was calculated at × 40 microscopic magnification by measuring the distance between the dermal–epidermal junction and the derma–subcutaneous fat junction (μm; as indicated by H&E staining in (a) in five randomly selected fields for each skin section. (c) IHC for α-SMA and LTBP-4 in normal tissue, LSc, and SSc. More positive cells were observed in the LSc and SSc group compared with control one. Black arrow indicates α-SMA-positive staining and LTBP-4-positive staining. The length of bars in the figures with an original magnification of × 100 are 200 μm, and the length of bars in the figures with an original magnifications of × 400 is 100 μm. Bars show the mean±s.e.m. results in each group. ***P<0.0001. H&E, hematoxylin/eosin; IHC, immunohistochemistry; LTBP-4, latent TGF-β-binding protein 4; LSC, localized scleroderma; α-SMA, α-smooth muscle actin; SSC,systemic scleroderma.
The Expression of LTBP-4 and TGF-β was Increased in the Plasma From SSc Patients
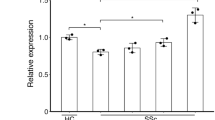
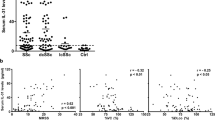
Plasma from healthy individuals, and LSc and SSc patients were collected to detect LTBP-4 and TGF-β levels in the peripheral blood. The results from ELISA assays showed that LTBP-4 levels in SSc patients were significantly higher than in controls (1215±100.2 vs 542.8±41.7 ng/ml, P<0.0001), while no significant difference was detected between LSc patients and controls (671.5±60.6 vs 542.8±41.7 ng/ml, P=0.08; Figure 3a). There was also a significant increase in SSc patients compared with the LSc group (1215±100.2 vs 671.5±60.6 ng/ml, P<0.0001). Further clinical correlation with the plasma LTBP-4 level was examined in SSc patients. Higher plasma levels were detected in SSc patients with esophagus involvement (1390±134.4 vs 940.7±127.0 ng/ml, P=0.0269; Figure 3b) and pulmonary fibrosis (1462±137.3 vs 892.8±113.4 ng/ml, P=0.0037; Figure 3c). No obvious difference was observed between plasma LTBP-4 level and other clinical symptoms, including Raynaud's phenomenon, arthralgia, and heart involvement (Figure 3d–f). Further receiver operating characteristics (ROC) curve of LTBP-4 to identify SSc patients provided a sensitivity of 93% with a specificity of 67%, and the area under curve (AUC) was 0.86 (Supplementary Figure 3).
The protein level of LTBP-4 in the plasma was elevated in SSc patients. (a) LTBP-4 level detected by ELISA in plasma samples from patients with SSc, LSc, and healthy controls. (b) LTBP-4 level was elevated in the plasma of SSc patients with esophagus involvement. (c) LTBP-4 level was elevated in the plasma of SSc patients with pulmonary fibrosis. (d–f) No obvious difference was detected in SSc patients with Raynaud’s phenomenon, arthralgia, and heart involvement. Results represent the mean±s.e.m. in each group. ***P<0.0001, **P<0.01, *P<0.05. LTBP-4, latent TGF-β-binding protein 4; LSC, localized scleroderma; SSC, systemic scleroderma.
As LTBP-4 facilitates latent TGF-β secretion activation, TGF-β levels were also detected in the peripheral blood. Similar to LTBP-4, there was a remarkable increase of TGF-β levels in the SSc group compared with normal controls (1.5±0.2 vs 0.7±0.1 ng/ml, P=0.0031), while no obvious difference was observed in LSc when compared with the control group (1.0±0.1 vs 0.7±0.1 ng/ml, P=0.1229). Simultaneously, no statistical difference was detected between SSc and LSc groups, although SSc patients showed a higher mean level in plasma TGF-β (1.5±0.2 vs 1.0±0.1 ng/ml, P=0.0648; Figure 4a). In the SSc group, patients with esophagus involvement and pulmonary fibrosis also showed a higher plasma TGF-β level (esophagus involvement, 1.9±0.3 vs 0.9±0.2 ng/ml, P=0.0426; pulmonary fibrosis, 2.0±0.4 vs 0.9 ±0.2 ng/ml, P=0.0212; Figure 4b and c). Further association of plasma LTBP-4 and TGF-β levels was analyzed, and the results showed a positive correlation in normal controls, LSc, and SSc patients (r2=0.385, P<0.0001; Figure 4d).
The protein level of TGF-β in the plasma was elevated in SSc patients. (a) TGF-β level detected by ELISA in plasma samples from patients with SSc, LSc, and healthy controls. (b) LTBP-4 level was elevated in the plasma of SSc patients with esophagus involvement. (c) LTBP-4 level was elevated in the plasma of SSc patients with pulmonary fibrosis. (d) Plasma TGF-β levels correlated positively with LTBP-4 levels in normal controls, and LSc and SSc patients (r=0.385, P<0.0001). Results represent the mean±s.e.m. in each group. **P<0.01, *P<0.05. LTBP-4, latent TGF-β-binding protein 4; LSc, localized scleroderma; SSc, systemic scleroderma; TGF-β, transforming growth factor-β.
Knockdown of LTBP-4 Downregulated the Expression of Collagen in SSc Skin Fibroblasts
Activated fibroblasts overexpress various ECM components, and type I collagen is one of the main components that contributes to tissue fibrosis in scleroderma patients.26 In order to explore the role of LTBP-4 in the regulation of collagen gene and protein expression, scleroderma skin fibroblasts were transfected with LTBP-4 siRNA and collected 48 h after transfection. RT-PCR analysis showed that LTBP-4, COL1A1, COL1A2, and COL3A1 mRNA levels were decreased by 75%, 84%, 82%, and 43%, respectively. Decrease of pro-fibrotic genes CTGF, FN-1, and PAI-1 was further detected by 76%, 75%, and 85%, respectively (Figure 5a). Western blotting indicated an apparent reduction on protein levels of LTBP-4 and type I collagen in siRNA knockdown skin fibroblasts compared with the normal control group (Figure 5b and c).
Transfection of LTBP-4 siRNA into scleroderma skin fibroblasts induces decreased collagen expression. (a) RT-PCR showed LTBP-4, COL1A1, COL1A2, and other fibrosis-related gene expression 48 h after knockdown treatment with LTBP-4 siRNA, and the mRNA levels were calculated using a relative ratio to GAPDH. (b) LTBP-4 and collagen type I protein expression levels were assessed by western blot and GAPDH was used as a reference control for equal protein loading. (c) Densitometry data of the protein were analyzed in two groups. Bars represented mean±s.e.m. in each group. ***P<0.0001, **P<0.01, *P<0.05. LTBP-4, latent TGF-β-binding protein 4; RT-PCR, real-time PCR.
Knockdown of LTBP-4 Downregulated TGF-β Levels in the Media of SSc Skin Fibroblasts
It has been shown that LTBPs induced secretion and activation of latent TGF-β in ECM. To further investigate the possible relationship between LTBP-4 and TGF-β in the fibrotic process, the secretion of TGF-β was measured in the supernatant of scleroderma skin fibroblasts using an ELISA assay. After 48 h, a reduction of TGF-β levels was observed in the LTBP-4 siRNA treatment group compared with the negative control group (0.007±0.002 vs 0.069±0.019 ng/ml, P=0.0087; Figure 6a). This result further suggested that the downregulation of LTBP-4 greatly reduced extracellular TGF-β levels and may have led to a reduction of downstream collagen expression and skin fibrosis.
Knockdown of LTBP-4 decreases TGF-β levels in the supernatant and suppresses phosphorylation of SMAD2 and SMAD3 in scleroderma skin fibroblasts. (a) The TGF-β level in the conditioned media of LTBP-4 knockdown fibroblasts and control group was tested by an ELISA kit and represented as mean±s.e.m. (b) Scleroderma skin fibroblasts were transfected with SBE luciferase reporter construct along with SV40 control vector and cultured in the absence or presence of LTBP-4 siRNA; the results are presented as mean±s.e.m. (c) SMAD2/3 and pSMDAD2/3 protein expression levels were assessed by western blot and GAPDH was used as a reference control for equal protein loading. (d) Densitometry data of the protein were analyzed in two groups represented as mean±s.e.m. **P<0.01. LTBP-4, latent TGF-β-binding protein 4; SBE, SMAD-binding element; TGF-β, transforming growth factor-β.
SMAD2 and SMAD3 Phosphorylations were Inhibited in LTBP-4 Knockdown Scleroderma Skin Fibroblasts
As TGF-β regulates collagen gene expression through the TGF-β/SMAD pathway in scleroderma,27 the effects of LTBP-4 on the TGF-β/SMAD signaling pathway and collagen production need to be further detected. In our study we evaluated intracellular SMAD expression in LTBP-4 knockdown scleroderma skin fibroblasts. As shown in Figure 6b, the relative luciferase activity of SBE was significantly downregulated in the LTBP-4 siRNA group compared with the control group (0.058±0.002 vs 0.080±0.004). In addition, western blotting showed an obviously decreased phosphorylation of the SMAD2/3 protein level in the siRNA knockdown group compared with the control group. In contrast to phosphorylated protein, the level of total SMAD2/3 protein was similar in control and siRNA- treated fibroblasts (Figure 6c). These changes demonstrate that knockdown of LTBP-4 may alleviate skin fibrosis through the inhibition of the TGF-β/SMAD pathway.
Discussion
Scleroderma tissue is characterized by excessive production and deposition of collagen, but the underlying mechanism is still unknown. Previous in vivo studies have determined that the role of LTBP-4 in the pathogenesis of lung fibrosis is to affect the secretion of TGF-β and biosynthesis of collagen,19 suggesting that LTBP-4 might be a fibrosis-related gene. The goal of the present research was to determine the role of LTBP-4 in the fibrotic process of scleroderma.
As shown in Figure 2, a significant increase of LTBP-4 along with collagen was observed in the skin tissue of scleroderma patients, suggesting that LTBP-4 may be associated with collagen synthesis. The importance of LTBP-4 in collagen synthesis was further demonstrated in LTBP-4 knockdown fibroblasts, which showed significant decreases in type I and type III collagen expression. The upregulation of LTBP-4 protein levels was also detected in the skin of a BLM-induced scleroderma mouse model, in which increased expression of collagen was significant. Consistently, upregulation of LTBP-4, as well as the mRNA levels of type I and III collagens, were also detected in the fibroblasts of BLM mice. Taken together, these results illustrate that LTBP-4 may participate in the fibrotic process of scleroderma by increasing the collagen production.
LTBP-4 is a fibrosis-related gene whose main function is to facilitate TGF-β secretion and deposition in ECM.28 TGF-β has been widely implicated in the fibrotic mechanism of scleroderma.2 In our study, SSc patients showed a higher plasma TGF-β level compared to the controls, which is consistent with a previous report.29 Importantly, plasma LTBP-4 levels were positively correlated with TGF-β in SSc patients. This could be explained by the fact that LTBP-4 might facilitate TGF-β secretion. Besides, elevated plasma LTBP-4 and TGF-β levels also showed a significant correlation with pulmonary fibrosis and esophagus involvement in SSc patients. This result is consistent with Murphy-Ullrich’s report of increased LTBP-4 levels in BALF from BLM-induced mouse model of lung fibrosis.19 The higher plasma LTBP-4 and TGF-β level in SSc patients with pulmonary fibrosis suggests that these two molecules may have important roles in internal organ fibrosis, and may determine severity of scleroderma patients. As previously reported, pulmonary fibrosis is considered the most serious visceral involvement, and interstitial lung disease is the major cause of mortality in SSc patients.30
For the LSc patients, the plasma LTBP-4 and TGF-β levels were lower compared with SSc patients, and no obvious difference was detected between LSc and the normal group. As TGF-β is the main pro-fibrotic cytokine-regulating collagen production, the relative low level of LTBP-4 and TGF-β in LSc might explain why the disease primarily affects the skin and subcutaneous tissues without organ involvement.31 Also, ROC curve analysis indicated that the serum LTBP-4 levels might serve as a useful biomarker for differentiating SSc patients from normal subjects with an AUC of 0.86. Taken together, increased plasma LTBP-4 might induce skin and organ fibrosis through the function of TGF-β in SSc patients. It may also act as a useful biomarker to distinguish SSc patients.
TGF-β has a pre-eminent role in the fibrotic process of scleroderma. Previous studies have shown that TGF-β could exacerbate skin fibrosis of scleroderma through myofibroblast activation and endothelial–mesenchymal transition,32, 33 while in the upstream of TGF-β signaling pathway LTBP-4 has been proved to regulate the export of TGF-β into ECM.34 In our study, a decreased level of TGF-β was observed in the supernatant of LTBP-4-knockdown fibroblasts, suggesting that LTBP-4 correlates with the export of TGF-β in SSc patients. We also observed a prominent decrease of SBE luciferase activity and phosphorylated SMAD2/3 protein expression in LTBP-4-knockdown fibroblasts, suggesting inhibition of SMAD2/3 activation and TGF-β/SMAD signaling. Moreover, previous studies indicated an elevated TGF-β receptor level in dermal fibroblasts of SSc patients, which promotes the activation of TGF-β/SMAD signaling, and treatment with TGF-β receptor siRNA inhibited the activation.35, 36 Thus, the decreased activity of TGF-β/SMAD signaling in our study might be caused by both decreased amount of TGF-β in the supernatant and TGF-β receptors. Further studies should be performed to explore more potential pathogenesis for LTBP-4 in scleroderma, including its effect on TGF-β receptor.
In summary, the present results suggest that LTBP-4 protein level is increased in plasma and skin tissue of scleroderma patients, and inhibition of LTBP-4 reduces collagen synthesis and alleviates fibrosis through the TGF-β/SMAD pathway. In addition, LTBP-4 may also serve as a biomarker to differentiate SSc from LSc patients. The results of our study demonstrate the importance of LTBP-4 in scleroderma, and its correlation with TGF-β/SMAD signaling. It provides evidence of antifibrotic effort of LTBP-4 inhibition that may be further explored as a potential therapeutic approach for scleroderma.
References
Careta MF, Romiti R . Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol 2015; 90: 62–73.
Varga J, Pasche B . Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol 2009; 5: 200–206.
Budzynska-Wlodarczyk J, Michalska-Jakubus MM, Kowal M et al, Evaluation of serum concentrations of the selected cytokines in patients with localized scleroderma. Postepy Dermatol Alergol 2016; 33: 47–51.
Bharadwaj S, Tandon P, Gohel T et al, Gastrointestinal manifestations, malnutrition, and role of enteral and parenteral nutrition in patients with scleroderma. J Clin Gastroenterol 2015; 49: 559–564.
Gasse P, Mary C, Guenon I et al, IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007; 117: 3786–3799.
Akgedik R, Akgedik S, Karamanli H et al, Effect of resveratrol on treatment of bleomycin-induced pulmonary fibrosis in rats. Inflammation 2012; 35: 1732–1741.
Todorovic V, Rifkin DB . LTBPs, more than just an escort service. J Cell Biochem 2012; 113: 410–418.
Verrecchia F, Mauviel A, Farge D . Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev 2006; 5: 563–569.
Gordon KJ, Blobe GC . Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim Biophys Acta 2008; 1782: 197–228.
Kantola AK, Ryynanen MJ, Lhota F et al, Independent regulation of short and long forms of latent TGF-beta binding protein (LTBP)-4 in cultured fibroblasts and human tissues. J Cell Physiol 2010; 223: 727–736.
Zilberberg L, Todorovic V, Dabovic B et al, Specificity of latent TGF-beta binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol 2012; 227: 3828–3836.
Bultmann I, Conradi A, Kretschmer C et al, Latent transforming growth factor beta-binding protein 4 is downregulated in esophageal cancer via promoter methylation. PLoS ONE 2013; 8: e65614.
Kretschmer C, Conradi A, Kemmner W et al, Latent transforming growth factor binding protein 4 (LTBP4) is downregulated in mouse and human DCIS and mammary carcinomas. Cell Oncol 2011; 34: 419–434.
Chan SH, Yee KJ, Chan KW et al, The ECM protein LTBP-2 is a suppressor of esophageal squamous cell carcinoma tumor formation but higher tumor expression associates with poor patient outcome. Int J Cancer 2011; 129: 565–573.
Chen H, Ko JM, Wong VC et al, LTBP-2 confers pleiotropic suppression and promotes dormancy in a growth factor permissive microenvironment in nasopharyngeal carcinoma. Cancer Lett 2012; 325: 89–98.
Mauel S, Kruse B, Etschmann B et al, Latent transforming growth factor binding protein 4 (LTBP-4) is downregulated in human mammary adenocarcinomas in vitro and in vivo. APMIS 2007; 115: 687–700.
Wang X, Zhang Y, Nilsson CL et al, Association of chromosome 19 to lung cancer genotypes and phenotypes. Cancer Metastasis Rev 2015; 34: 217–226.
Bultmann-Mellin I, Conradi A, Maul AC et al, Modeling autosomal recessive cutis laxa type 1C in mice reveals distinct functions for Ltbp-4 isoforms. Dis Model Mech 2015; 8: 403–415.
Zhou Y, Koli K, Hagood JS et al, Latent transforming growth factor-beta-binding protein-4 regulates transforming growth factor-beta1 bioavailability for activation by fibrogenic lung fibroblasts in response to bleomycin. Am J Pathol 2009; 174: 21–33.
Sato S, Ihn H, Kikuchi K et al, Antihistone antibodies in systemic sclerosis. Association with pulmonary fibrosis. Arthritis Rheum 1994; 37: 391–394.
Wu T, Chu H, Tu W et al, Dissection of the mechanism of traditional Chinese medical prescription-Yiqihuoxue formula as an effective anti-fibrotic treatment for systemic sclerosis. BMC Complement Altern Med 2014; 14: 224.
Yoshizaki A, Yanaba K, Yoshizaki A et al, Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum 2010; 62: 2476–2487.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25: 402–408.
Wang JC, Sonnylal S, Arnett FC et al, Attenuation of expression of extracellular matrix genes with siRNAs to Sparc and Ctgf in skin fibroblasts of CTGF transgenic mice. Int J Immunopathol Pharmacol 2011; 24: 595–601.
Wang JC, Lai S, Guo X et al, Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther 2010; 12: R60.
Kudo H, Wang Z, Jinnin M et al, EBI3 downregulation contributes to type I collagen overexpression in scleroderma skin. J Immunol 2015; 195: 3565–3573.
Jinnin M . Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol 2010; 37: 11–25.
Kantola AK, Keski-Oja J, Koli K . Fibronectin and heparin binding domains of latent TGF-beta binding protein (LTBP)-4 mediate matrix targeting and cell adhesion. Exp Cell Res 2008; 314: 2488–2500.
Vettori S, Cuomo G, Iudici M et al, Early systemic sclerosis: serum profiling of factors involved in endothelial, T-cell, and fibroblast interplay is marked by elevated interleukin-33 levels. J Clin Immunol 2014; 34: 663–668.
Gazdhar A, Lebrecht D, Roth M et al, Time-dependent and somatically acquired mitochondrial DNA mutagenesis and respiratory chain dysfunction in a scleroderma model of lung fibrosis. Sci Rep 2014; 4: 5336.
Fett N, Werth VP . Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol 2011; 64: 217–228 229-230.
Cipriani P, Di Benedetto P, Ruscitti P et al, Macitentan inhibits the transforming growth factor-beta profibrotic action, blocking the signaling mediated by the ETR/TbetaRI complex in systemic sclerosis dermal fibroblasts. Arthritis Res Ther 2015; 17: 247.
Cipriani P, Di Benedetto P, Ruscitti P et al, The endothelial-mesenchymal transition in systemic sclerosis is induced by endothelin-1 and transforming growth factor-beta and may be blocked by macitentan, a dual endothelin-1 receptor antagonist. J Rheumatol 2015; 42: 1808–1816.
Cutroneo KR, White SL, Phan SH et al, Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol 2007; 211: 585–589.
Pannu J, Gore-Hyer E, Yamanaka M et al, An increased transforming growth factor beta receptor type I:type II ratio contributes to elevated collagen protein synthesis that is resistant to inhibition via a kinase-deficient transforming growth factor beta receptor type II in scleroderma. Arthritis Rheum 2004; 50: 1566–1577.
Pannu J, Gardner H, Shearstone JR et al, Increased levels of transforming growth factor beta receptor type I and up-regulation of matrix gene program: a model of scleroderma. Arthritis Rheum 2006; 54: 3011–3021.
Acknowledgements
This study was partially supported by grants from the National Science Foundation of China (81470254, 81328001, 81270120), International S&T Cooperation Program of China (2013DFA30870), US NIH NIAID U01 (1U01AI090909), and the 111 Project (B13016) from Ministry of Education (MOE). Computational support was provided by the High-End Computing Center located at Fudan University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
Scleroderma is a fibrosis-related disorder in which TGF-β plays a central role and latent TGF-β binding protein 4 (LTBP-4) affects the secretion of latent TGF-β to the extracellular matrix. Increased expression of LTBP-4 is found in serum and skin tissues of scleroderma patients, and its knockdown downregulates the secretion of TGF-β and activation of TGF-β/SMAD signaling. LTBP-4 plays roles in scleroderma by regulating TGF-β secretion.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, J., Liu, Q., Wang, L. et al. Increased expression of latent TGF-β-binding protein 4 affects the fibrotic process in scleroderma by TGF-β/SMAD signaling. Lab Invest 97, 591–601 (2017). https://doi.org/10.1038/labinvest.2017.20
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2017.20
This article is cited by
-
The pan HDAC inhibitor Givinostat improves muscle function and histological parameters in two Duchenne muscular dystrophy murine models expressing different haplotypes of the LTBP4 gene
Skeletal Muscle (2021)
-
ILDGDB: a manually curated database of genomics, transcriptomics, proteomics and drug information for interstitial lung diseases
BMC Pulmonary Medicine (2020)
-
TGFβ-mediated expression of TGFβ-activating integrins in SSc monocytes: disturbed activation of latent TGFβ?
Arthritis Research & Therapy (2020)
-
Histomorphological, VEGF and TGF-β immunoexpression changes in the diabetic rats’ ovary and the potential amelioration following treatment with metformin and insulin
Journal of Molecular Histology (2020)
-
DHEA-induced ovarian hyperfibrosis is mediated by TGF-β signaling pathway
Journal of Ovarian Research (2018)