Abstract
Endoplasmic reticulum (ER) stress, resulting from the accumulation of misfolded and/or unfolded proteins in ER membranes, is involved in the pathogenesis of diabetic nephropathy (DN). The aim of this study was to investigate the role of ER stress inhibitors ursodeoxycholic acid (UDCA) and 4-phenylbutyrate (4-PBA) in the treatment of DN in db/db mice. Findings have revealed that diabetic db/db mice were more hyperglycemic than their non-diabetic controls, and exhibited a marked increase in body weight, water intake, urine volume, fasting plasma glucose, systolic blood pressure, glucose and insulin tolerance. UDCA (40 mg/kg/day) or 4-PBA (100 mg/kg/day) treatment for 12 weeks resulted in an improvement in these biochemical and physical parameters. Moreover, UDCA or 4-PBA intervention markedly decreased urinary albuminuria and attenuated mesangial expansion in diabetic db/db mice, compared with db/db mice treated with vehicle. These beneficial effects of UDCA or 4-PBA on DN were associated with the inhibition of ER stress, as evidenced by the decreased expression of BiP, phospho-IRE1α, phospho-eIF2α, CHOP, ATF-6 and spliced X-box binding protein-1 in vitro and in vivo. UDCA or 4-PBA prevented hyperglycemia-induced or high glucose (HG)-induced apoptosis in podocytes in vivo and in vitro via the inhibition of caspase-3 and caspase-12 activation. Autophagy deficiency was also seen in glomeruli in diabetic mice and HG-incubated podocytes, exhibiting decreased expression of LC3B and Beclin-1, which could be restored by UDCA or 4-PBA treatment. Taken together, our results have revealed an important role of ER stress in the development of DN, and UDCA or 4-PBA treatment may be a potential novel therapeutic approach for the treatment of DN.
Similar content being viewed by others
Main
Type 2 diabetic mellitus is a global problem that threatens people around the world. A 2010 national survey reported that diabetes affects approximately 113.9 million of the Chinese population,1 with an increase from 9.7% in 2007 to 11.6% in 2010.2 In addition, overall prevalence of chronic kidney disease was 10.8% in China, according to a National Survey from 2007–2010.3 Diabetic nephropathy (DN) is recognized as the leading cause of end-stage renal disease.4 Although attempts on the prevention of the development and progression of DN through strict control of blood glucose and blood pressure have been made, these treatments are far from satisfactory.5 Therefore, it is important to develop novel therapeutics that allow for the prevention and retardation of DN.
Endoplasmic reticulum (ER) stress is involved in protein synthesis and degradation systems, and is fundamentally required for physiological activities.6 ER stress occurs when homeostasis is disturbed. It can be induced by hyperglycemia, reactive oxygen species (ROS), free fatty acids and other mediators in the presence of diabetes,7 with the accumulation of unfolded proteins in ER.8 Previous evidences have demonstrated that ER stress may contribute to the development of DN.9, 10, 11 Autophagy, an essential process for basal homeostasis, acts as a mechanism to keep homeostatic balance by degrading damaged cellular organelles and proteins.12 Defective autophagy accelerates the irreparable progression of DN in mice.13 However, the role of ER stress and autophagy in DN has not been clearly elucidated.
Podocytes, or known as glomerular visceral epithelial cells, maintain the structure and function of the glomerular filtration barrier. Podocyte loss is found in patients with DN or micro-albuminuria14, 15 In fact, the loss of podocytes is one of the earliest features of DN that predicts its progressive course.16 However, molecular mechanisms that underlie the loss of podocytes in DN remain poorly understood.
Bile acids have recently emerged as key metabolic regulators of glucose and lipid metabolism. Ursodeoxycholic acid (UDCA), a major component of black bear bile, has been used for over 3000 years for the treatment of live diseases and visual system disorders,17, 18, 19 because of its beneficial effects on hepatic steatosis, insulin resistance and enhancement of ER adaptive capacity.20 4-Phenylbutyrate (4-PBA) is a low molecular weight chemical chaperone that has been used for the treatment of urea cycle disorders. It has been reported that 4-PBA can suppress ER stress.21, 22 Ozcan et al20 found that both UDCA and 4-PBA alleviated ER stress, which resulted in the normalization of hyperglycemia and restoration of systemic insulin sensitivity, thereby revealing their potential benefits in the treatment of type 2 diabetes. Therefore, we hypothesize that UDCA and 4-PBA may prevent kidney damage in type 2 diabetic mellitus through the inhibition of ER stress.
This study aims to evaluate the role of ER stress on renal morphology and function in diabetic db/db mice in vivo or high glucose (HG)-induced podocytes in vitro. We have also investigated the renoprotective effects of UDCA and 4-PBA against DN and its underlying mechanisms.
MATERIALS AND METHODS
Animals and Grouping
Male diabetic mice at 6 weeks of age with a homozygous mutation of the leptin receptor (C57BLKS/J-leprdb/leprdb) were used as a DN model. Age-matched non-diabetic (db/m) mice were purchased from the Model Animal Research Center of Nanjing University in China and served as controls. Mice were given free access to food and water, and were maintained under a 12/12 h light–dark cycle with controlled temperature (23±2 °C) and humidity (55±5%). This experimental protocol was approved by the Ethics Committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine. After 2 weeks of adaptation, db/m mice (n=12) were administrated intragastrically with vehicle (0.5% carboxymethyl cellulose solution). Thirty-six db/db mice were randomly divided into three groups: vehicle-treated diabetic group (n=12), UDCA-treated diabetic group (n=12), treated with 40 mg/kg/day UDCA (Sigma-Aldrich, MO, USA) in vehicle. 4-PBA-treated diabetic group (n=12), treated with 100 mg/kg/d 4-PBA (Santa Cruz Biotechnology, CA, USA) in vehicle.23, 24 All mice were treated for 12 weeks. Body weight, blood glucose, urine volume, water intake and blood pressure were recorded every 4 weeks. Blood pressure was measured in mice by tail-cuff plethysmography (Shanghai AlcottBiotech, Shanghai, China), and 24-h urine samples were collected to measure urinary albumin excretion by a urinary albumin kit (Abcam, Cambridge, UK) at the end of the experiments. All mice underwent 1-day acclimatization period in metabolic cages before urine collection. Mice were killed at the end of the experiments. Fasting blood samples and kidney tissues were collected for further analysis.
Intraperitoneal Glucose Tolerance and Insulin Tolerance Tests
Intraperitoneal glucose tolerance test (GTT) and insulin tolerance test (ITT) were performed as previously described.25 After an overnight fast, GTT or ITT were performed by intraperitoneal injection of glucose (2 g/kg) or insulin (0.75 U/kg) in sterile saline, respectively. Blood glucose was measured at 0, 15, 30, 60, 90 and 120 min by an OneTouch Ultra blood glucose meter (OMRON, Kyoto, Japan).
Serum Insulin and Urinary Albumin Excretion
At the end of the experiments, blood samples were collected and centrifuged, and serum samples were stored at −80 °C. Serum insulin and urinary albumin excretion were determined by ELISA kits (Millipore, MA, USA).
Histopathological Analysis and Immunohistochemistry
Kidney tissues were fixed in 4% paraformaldehyde and embedded in paraffin. Paraffin sections (5 μm) were stained with 0.5% periodic acid and Schiff (PAS) to evaluate the glycogen content as previously reported.26 Twenty to thirty glomeruli from each mouse were randomly selected. Semiquantitative scoring of glomerular sclerosis in PAS-stained slides was performed using method described previously.27 Section immunostaining was performed using primary antibody against Wilim’s Tumor-1 or LC3B (Abcam) as previously described.28
Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
TUNEL assay was performed by using an In Situ Cell Death Detection kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. At least 100 glomeruli were examined. All slides were examined by an investigator who was unaware of the group assignment of mice.
Cell Culture
Conditionally immortalized mouse podocytes were provided by Professor Niansong Wang (Shanghai Sixth People’s Hospital, China), which were originally provided by Dr Peter Mundel (Division of Nephrology, Massachusetts General Hospital, Harvard University). Cells were conducted as previously described.29 Differentiated podocytes were cultured for 24 h in RMPI-1640 supplemented with 1% fetal calf serum before being exposed to various experimental conditions. Cells were divided into the following groups: normal glucose (5 mM) group, mannitol group, incubated in normal glucose with 25 mM of D-mannitol (Sigma-Aldrich), HG group, incubated in RPMI-1640 containing 30 mM of d-glucose (Sigma-Aldrich), UDCA (50 μM) group and 4-PBA (2.5 mM) group that were pretreated with UDCA or 4-PBA for 1 h followed by stimulation with 30 mM d-glucose for 24 or 72 h. All experiments were performed in triplicate.
Apoptosis Detection by ANNEXIN-V/Propidium Iodide (PI) Staining
Podocytes apoptosis was determined using an Annexin-V/PI double staining kit (BioVision, CA, USA), as previously reported.30 Samples were analyzed by flow cytometry (BD FACSCalibur, NJ, USA).
Green Fluorescent Protein (GFP)-LC3B Transfection
Podocytes were plated onto glass coverslips at a density of 2 × 105 in a six-well plates and transfected with Premo™ Autophagy Sensor LC3B-GFP (Invitrogen, CA, USA) for 48 h according to the manufacturer’s instructions. GFP-LC3-transfected cells were treated under control and experimental conditions, and observed with a confocal microscope (LAM 880, Zeiss, Germany).
Western Blot Analysis
Primary antibodies were used as follows: rabbit anti-ATF-6, WT-1, LC3B and X-BP1 antibodies (Abcam); BiP, CHOP, caspase-12, caspase-3, LC3 A/B I/II and GAPDH antibodies (Cell Signaling Technology, MA, USA); phospho-IRE1α (Novus Biologicals, CO, USA); phospho-PERK (Thermo Fisher Scientific, MA, USA). Kidney cortex or cultured podocytes were lysed in lysis buffer with a sonicator and centrifuged at 14 000 × g for 5 min at 4 °C. In all, 40 μg of total protein samples were separated by 8–12% gel electrophoresis and electrotransferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% bovine serum albumin (BSA) for 1 h and incubated with primary antibodies overnight at 4 °C. Then the membranes were washed in Tris-buffered NaCl solution with Tween 20 (TBST, containing 0.1% Tween 20), and then incubated with horseradish peroxidase-conjugated secondary antibodies in 5% BSA for 1 h. Density of the corresponding bands was measured by Bio-Rad VersDoc imaging system using chemiluminescence detection reagents (Thermo Fisher Scientific). The data were analyzed with Bio-Rad Quantity One software (Bio-Rad, CA, USA), and corrected by reference to the value of GAPDH.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) and Student–Newman–Keuls test for multiple comparisons, and Student’s t-test for two conditions using GraphPad Prism software (version 5, GraphPad Software, CA, USA). Data were expressed as mean and standard deviation (mean±s.d.). P<0.05 was considered statistically significant.
RESULTS
Effect of UDCA or 4-PBA on Biochemical and Physical Parameters
During the observation period, diabetic db/db mice displayed a marked increase in body weight, water intake, urine volume, fasting plasma glucose and systolic blood pressure compared with non-diabetic db/m mice, as shown in Figure 1. There were no significant differences in body weight between mice in the UDCA or 4-PBA group and untreated db/db mice until 12 weeks of intervention. Moreover, UDCA or 4-PBA significantly reduced water intake, fasting plasma glucose level and systolic blood pressure in mice after 8 weeks of treatment, whereas 4-PBA reduced urine volume in mice after 4 weeks of treatment (P<0.001).
Effect of ursodeoxycholic acid (UDCA) or 4-PBA on biochemical and physical parameters in db/db and db/m mice. Thirty-six diabetic db/db mice were administered intragastrically with vehicle, 40 mg/kg/d UDCA in vehicle or 100 mg/kg/d 4-PBA in vehicle (n=12 each group) for 12 weeks. Twelve db/m mice were selected as the control group and were administered intragastrically with vehicle. (a) Body weight; (b) blood glucose; (c) urine volume; (d) water intake and (e) systolic blood pressure were recorded every 4 weeks. **P<0.001, compared with db/m mice, ##P<0.001, compared with db/db mice.
Effect of UDCA or 4-PBA on Glucose Tolerance and Insulin Sensitivity
As illustrated in Figure 2a, there was a continuous increase in blood glucose at all the time points during the GTT in db/db mice compared with the normal mice. From the AUC of the four groups, we could observe an obvious decrease in glucose in 4-PBA-treated group compared with the untreated diabetic group (P<0.001; Figure 2c). In addition, both UDCA- or 4-PBA-treated group showed a marked difference in the rapid removal of blood glucose versus the db/db group in ITT (P<0.001; Figure 2d).
Ursodeoxycholic acid (UDCA) and 4-PBA improved glucose tolerance and insulin sensitivity in db/db mice after 12-week treatment. Diabetic db/db mice were treated with vehicle, 40 mg/kg/d UDCA in vehicle or 100 mg/kg/d 4-PBA in vehicle, and db/m mice treated with vehicle for 12 weeks. Blood glucose levels during intraperitoneal GTTs (a) and ITTs (b) in db/m and db/db mice were also expressed as AUC (c, d) measured after 12 weeks of intervention. **P<0.001, compared with db/m mice, #P<0.05, ##P<0.001, compared with db/db mice (n=6).
Effect of UDCA or 4-PBA on Serum Insulin and Urinary Albumin Excretion
Diabetic db/db mice exhibited a significantly higher level of serum insulin than db/m mice. Interestingly, both UDCA and 4-PBA treatment for 12 weeks showed a powerful effect in lowering serum insulin levels in diabetic mice.
Albuminuria is an indicator that precedes and predicts the development of DN. In this study, vehicle-treated diabetic rats exhibited a remarkable increase in urinary albumin, compared with control db/m mice. Treatment with UDCA or 4-PBA significantly decreased urinary albumin excretion in diabetic db/db mice (Figure 3; P<0.05 and P<0.001, respectively).
Effect of UDCA or 4-PBA on Kidney Morphology
Mesangial expansion is a histological feature of DN, which results from the excessive accumulation of extracellular matrix proteins. Here, we examined whether UDCA or 4-PBA treatment could reduce mesangial expansion in diabetic db/db mice. As shown in Figures 4a and b, histological findings showed a significant mesangial expansion in diabetic mice. UDCA or 4-PBA markedly attenuated mesangial expansion and glomerulosclerosis in diabetic db/db mice compared with mice treated with vehicle (P<0.001).
Ursodeoxycholic acid (UDCA) or 4-PBA restored autophagy and alleviated mesangial matrix expansion and apoptosis in diabetic db/db mice. (a) Representative images of PAS-stained kidney sections from vehicle-treated db/m, vehicle-treated db/db, 40 mg/kg UDCA-treated db/db and 100 mg/kg 4-PBA-treated db/db mice. (b) Semiquantitative glomerulosclerotic scores of each group, determined from 20 to 30 glomeruli in each mouse. (c) Representative glomeruli immunostained with TUNEL and (d) the number of TUNEL-positive cells within the glomeruli obtained from all groups. (e) Representative glomeruli immunostained with Wilm’s Tumor-1 (WT-1). (f) The number of WT-1-positive nulcei (brown nuclei staining) per glomerulus. (g) Representative glomeruli immunostained with LC3B. **P<0.001, compared with db/m mice, ##P<0.001, compared with db/db mice (n=12).
The number of apoptotic cells within the glomeruli was marked increased in db/db group compared with UDCA or 4-PBA-treated mice (Figures 4c and d; P<0.001). Podocyte apoptosis/depletion is usually associated with the onset of DN.31 In this study, diabetic db/db mice exhibited significant podocyte loss compared with non-diabetic db/m mice. However, the number of apoptotic podocytes significantly decreased in UDCA- or 4-PBA-treated diabetic mice, compared with vehicle-treated diabetic db/db mice (Figures 4f and g; P<0.001). These data suggest that UDCA or 4-PBA is a potential novel therapeutic approach for the treatment of DN.
Effect of UDCA or 4-PBA on Autophagy in Glomeruli
We further explored the effect of autophagy in glomeruli by staining with anti-LC3B antibody, a marker of autophagy. Histological findings revealed that autophagosomes were distributed normally in the glomeruli of db/m mice. In contrast, vehicle-treated diabetic db/db mice exhibited a reduced number of autophagosomes. However, this autophagy deficiency in glomeruli in diabetic mice could be restored by UDCA or 4-PBA treatment (Figure 4g).
UDCA or 4-PBA Regulated HG-Inhibited Autophagy in Podocytes
To examine the role of autophagy in podocytes after HG exposure, western blot and immunohistochemistry were performed. Incubation with HG led to the formation of LC3 A/B II, the autophagic form of LC3 A/B, and Beclin-1, which markedly increased at 24 h and then decreased slightly thereafter (Figures 5b and c). However, UDCA or 4-PBA abolished HG-inhibited autophagy, as evidenced by an increase in GFP-LC3B green fluorescence and accumulation Beclin-1 by western blot analysis (Figure 5a, d and e).
Ursodeoxycholic acid (UDCA) or 4-PBA reversed high glucose (HG)-induced autophagy deficiency in podocytes. (a) UDCA or 4-PBA upregulated HG-inhibited autophagy in podocytes after 72-h incubation with HG for 72 h, as evidenced by an increase in GFP-LC3B green fluorescence (400 ×). (b) The expression of LC3 A/B I, II, and Beclin-1 proteins in podocytes were determined by western blot analysis. (c) Quantification of the protein levels of LC3 A/B II and Beclin-1, and corrected by reference to the value of GAPDH. (d) UDCA or 4-PBA increased the protein expression levels of LC3 A/B II and Beclin-1 at 72 h, determined by western blot analysis. (e) Quantification of the protein levels of LC3 A/B II and Beclin-1, and corrected by reference to the value of GAPDH. *P<0.05,**P<0.001, compared with control group. ##P<0.001, compared with HG group.
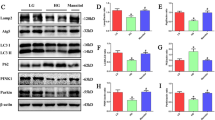
Effect of UDCA or 4-PBA on ER Stress in Diabetic Mice
Western blot analysis revealed significantly increased levels of ER stress markers in kidney tissues from diabetic db/db mice, including Bip, phospho-PERK, phospho-IRE1α, cleaved ATF-6, CHOP and spliced X-box binding protein-1 (XBP-1), suggesting hyperglycemia-induced ER stress in kidney (Figure 6). Both UDCA and 4-PBA could reduce the expression levels of BiP, phospho-PERK, phospho-IRE1α, cleaved ATF-6, CHOP and spliced XBP-1. Moreover, the activation of caspases, as shown by increased levels of cleaved caspase-3 and caspase-12, was observed in db/db mice. Interestingly, UDCA or 4-PBA could inhibit the activation of caspase-3 and caspase-12 in kidney tissues from diabetic db/db mice (P<0.05 and P<0.001, respectively).
Ursodeoxycholic acid (UDCA) and 4-PBA suppressed endoplasmic reticulum (ER) stress in diabetic db/db mice. (a) The expression levels of BiP, phospho-PERK, phospho-IRE1α, cleaved ATF-6 and GAPDH in kidney tissues from vehicle-treated db/m, vehicle-treated db/db, 40 mg/kg UDCA-treated db/db and 100 mg/kg 4-PBA-treated db/db mice, detected by western blot analysis, and quantification of these protein levels normalized by GAPDH levels (b). (c) The expression levels of spliced XBP-1, CHOP, caspase-12 and caspase-3 in kidney tissues, and quantification of these protein levels normalized by GAPDH levels (d). **P<0.001, compared with db/m mice, #P<0.05, ##P<0.001, compared with db/db mice (n=6).
Effect of HG on ER Stress and Apoptosis in Podocytes
We further investigated the effect of d-glucose on podocyte apoptosis, and determined whether UDCA and 4-PBA had an anti-apoptotic effect in vitro. Podocytes were incubated with 30 mM of d-glucose or mannitol (control). Results revealed that HG-induced podocyte apoptosis in a time-dependent manner. The apoptotic podocytes were significantly increased from 13.22% to 16.27%, 28.66%, 32.66%, 36.02% and 70.92% at 0, 12, 24, 48, 72 h, respectively (P<0.001; Figure 7). Next, podocytes were preincubated with 50 μM of UDCA or 2.5 mM of 4-PBA for 1 h and then treated with 30 mM of d-glucose for 72 h, and apoptosis was assessed by Annexin-V/PI staining. Findings showed that UDCA or 4-PBA could abolish d-glucose-induced apoptosis in podocytes (Figure 9; P<0.001).
High glucose (HG) triggered apoptosis in a time-dependent manner in podocytes. (a) Representative images of apoptotic podocytes detected by fluorescence activated cell sorter (FACS); (b) quantification of apoptotic podocytes. **P<0.001 compared with control. All experiments were performed in triplicate.
In vitro experiments were also performed to investigate whether ER stress was associated with HG-induced podocyte apoptosis. Culture in HG for 12–24 h remarkably induced the upregulation of ER stress-related proteins in podocytes, including BiP, phospho-PERK, phospho-IRE1α, CHOP and spliced XBP-1 (Figures 8a and b; P<0.001). Interestingly, cleaved ATF-6 was continuously increased from 12 to 72 h. Moreover, culture in HG for 12–72 h also resulted in the activation of apoptosis mediator, caspase-12 and caspase-3 (Figures 8c and d; P<0.001). Taken together, these data demonstrated that ER stress was involved in HG-induced podocyte apoptosis.
Effect of HG on ER stress in podocytes. (a) The expressions of BiP, phospho-PERK, phospho-IRE1α, cleaved ATF-6 and GAPDH in HG-incubated podocytes, detected by Western blotting analysis, and quantification of these protein levels normalized by GAPDH levels (b). (c) The expressions of spliced XBP-1, CHOP, caspase-12, and caspase-3 in HG-incubated podocytes, and quantification of these protein levels normalized by GAPDH levels (d). *P<0.05, **P<0.001, compared with control group. All experiments were performed in triplicate.
Effect of UDCA or 4-PBA on HG-induced apoptosis in podocytes. Podocytes were pre-treated with 50 μM UDCA or 2.5 mM 4-PBA 1 h followed by incubation with 30 mM D-glucose for another 72 h. (a) Representative images of apoptotic podocytes detected by fluorescence activated cell sorter (FACS); (b) quantification of apoptotic podocytes. **P<0.001, compared with control. ##P<0.001, compared with HG group. All experiments were performed in triplicate.
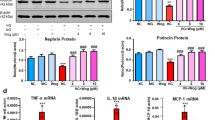
Effect of UDCA and 4-PBA on HG-Induced ER Stress in Podocytes
Protein levels of BiP, phospho-PERK, phospho-IRE1α, cleaved ATF-6, CHOP and spliced XBP-1; caspase-3 and caspase-12 in podocytes were significantly elevated after 24-h incubation with HG, and these effects could be abrogated by 50 μM of UDCA or 2.5 mM of 4-PBA, P<0.001 (Figure 10).
Effect of ursodeoxycholic acid (UDCA) or 4-PBA on high glucose (HG)-induced endoplasmic reticulum (ER) stress in podocytes. Podocytes were pretreated with 50 μM UDCA or 2.5 mM 4-PBA 1 h followed by incubation with 30 mM d-glucose for another 24 h. (a) The expression levels of BiP, phospho-PERK, phospho-IRE1α, ATF-6 and GAPDH in HG-induced podocytes, detected by western blot analysis, and its relative protein expression levels normalized by GAPDH levels (b). (c) The expression levels of spliced XBP-1, CHOP, caspase-12 and caspase-3 in high HG-induced podocytes, and and its relative protein expression levels normalized by GAPDH levels (d). **P<0.001, compared with control group. ##P<0.01, compared with HG treatment. All experiments were performed in triplicate.
DISCUSSION
The findings of this study demonstrated that ER stress was associated with hyperglycemia- or HG-induced podocyte apoptosis in vivo and in vitro, suggesting that ER stress was involved in the pathogenesis of DN. The inhibition of ER stress by UDCA or 4-PBA ameliorated insulin resistance, reduced podocyte apoptosis, restored autophagy deficiency and improved mesangial expansion in diabetic db/db mice, thus providing a potential therapeutic approach for the treatment of DN.
DN is a major microvascular complication of diabetes mellitus. In this study, db/db mice appeared to exhibit symptoms such as polydipsia, polyuria, hyperglycemia and obesity, especially in mice they exhibited increased albuminuria and mesangial matrix expansion, suggesting that db/db mice could be used as a model of progressive DN as previously reported.32, 33 Albuminuria, an indicator that implies to the impaired glomerular filtration barrier, is a dominant biochemical feature of developing DN. Progressive expansion of the mesangial matrix is a key histological appearance of DN.34 The db/db murine model of type 2 diabetes could develop renal dysfunction closely similar to that of humans. Thus, this model has been used in a number of studies for the research of DN.34, 35, 36 In this study, elevated levels of albuminuria and exacerbated histological alterations indicate damage in kidneys of diabetic db/db mice. Interestingly, UDCA or 4-PBA treatment could restore renal dysfunction and mesangial expansion in db/db mice, suggesting that both treatments effectively prevented the progression of DN.
Type 2 diabetes mellitus is a metabolic disease characterized by hyperglycemia, which mainly results from insulin resistance. Unrestrained endogenous glucose generation may contribute to fasting hyperglycemia, which is usually accompanied with peripheral insulin resistance and/or inadequate insulin release.37 Recently, podocytes, the key component of the glomerular filtration barrier, were found to be uniquely insulin sensitive in some manner, and they were able to take up glucose via the translocation of glucose transporters GLUT1 and GLUT4.38 In an insulin resistance state, the impaired capacity of glucose uptake for podocytes has been shown to provoke kidney dysfunction.39, 40 Several attempts have been made to overcome peripheral insulin resistance by amplifying insulin output.37 In this study, these findings have demonstrated that UDCA and 4-PBA ameliorated insulin resistance, and 4-PBA improved glucose tolerance in a diabetic db/db murine model.
Apoptosis triggered by environmental and generically susceptible factors contributes to progressive beta-cell dysfunction and insulin resistance in both type 1 and type 2 diabetes mellitus.41, 42 It has been reported that podocytes depletion or loss coincides with the onset of albuminuria.31 Hyperglycemia induces the generation of ROS via NADPH oxidase and activated caspases cascade, ultimately leading to podocyte apoptosis.16 Podocytes are terminally differentiated cells with limited capacity for cellular regeneration. Thus, podocyte apoptosis contributes to the irreversible decrease in the total number of podocytes. Therefore, the inhibition of podocyte apoptosis is beneficial for preventing albuminuria and delaying the progression of DN. In this study, we found that HG triggered podocyte apoptosis in a time-dependent manner, and UDCA or 4-PBA could abolish HG-induced apoptosis in podocytes in vitro. Similar effects of UDCA and 4-PBA were found in diabetic db/db mice, revealing that UDCA or 4-PBA could protect podocytes against HG-induced apoptosis.
ER stress is a tightly regulated cell process that has a vital role in regulating a number of physiological activities. ER stress triggers complex adaptive or proapoptotic signals defined as the unfolded protein response (UPR). In mammalian cells, there are three major arms of the UPR, including protein kinase RNA (PKR)-like ER kinase (PERK), inositol-requiring protein-1 (IRE1α) and activating protein transcription factor-6 (ATF-6). Under unstressed conditions, BiP/glucose-regulated protein 78 (BiP/GRP78), a central regulator of ER homeostasis, binds to proteins including PERK, IRE1α and ATF-6 and inhibites their activation. Mediators that are associated with diabetic mellitus increase the load of unfolded proteins and phosphorylation of PERK, IRE1α and ATF-6, resulting in the activation of caspases.10 In this study, we investigated the hypothesis that ER stress is partly responsible for podocyte apoptosis induced by HG. We observed that BiP, phosphor-PERK, phosphor-IRE1α, spliced XBP-1 and CHOP were increased in the cells exposed to HG (30 mM) at 12 and 24 h and decreased by 48 and 72 h. Cleaved ATF-6 was consistently elevated from 12 to 72 h, suggesting sustained ER stress activation. Our results were similar to other investigators.7, 43 Inagi et al44 demonstrated that podocytes treated with hyperglycemia did not upregulate ER stress markers. As mentioned in the section of methods in their study, podocytes were cultured in the presence of 4.5 mg/ml (25 mM) d- or l-glucose for 2 weeks. Therefore, the activation of ER stress in podocytes by HG depends on incubation time and the concentration of glucose. Both 4-PBA and UDCA have been successfully used to alleviate ER stress, restore glucose tolerance and improve insulin sensitivity.20, 45, 46 However, the effect of 4-PBA or UDCA on ER stress in DN remains un-elucidated. Our findings have shown that both UDCA and 4-PBA could suppress the UPR in podocytes in vitro, as evidenced by the decreased protein expression of BiP, phospho-PERK, phospho-IRE1α and cleaved ATF-6, which were upregulated by HG, suggesting that UDCA and 4-PBA could inhibit HG-induced ER stress in podocytes.
Excessive and sustained UPR leads to the activation of X-box binding protein-1, CCAAT/enhancer binding protein homologous protein (CHOP), caspase-12 and caspase-3, which in turn results to cell injury and death. When BiP dissociates from IRE1α, XBP-1 is spliced by activated IRE1α.10 The generation of ROS in the presence of diabetes mellitus enhances CHOP expression in podocytes.47 It has been reported that CHOP−/− mice on C57B1/J background preserve normal glucose tolerance and insulin sensitivity.48 Moreover, CHOP-deficient mice did not develop albuminuria and ER stress-induced apoptosis in proximal tubular cells.49 Caspase-12 is a member of the caspase family and is located in the ER.50 Caspase-12 is specifically activated by insults from ER stress, but not by some death stimuli. It has been reported that caspase-12-deficient mice are protected from tubular injury by tunicamycin.51 In this study, results have demonstrated that ER stress may be involved in hyperglycemia- or HG-induced apoptosis in podocytes in vivo and in vitro, as evidenced by the activation of XBP-1, CHOP, caspase-12 and caspase-3, implying the novel mechanism of podocyte apoptosis and pathogenesis of DN. Interestingly, UDCA and 4-PBA reduced podocyte apoptosis through the downregulation of ER stress-elevated proteins, such as XBP-1, CHOP, caspase-12 and caspase-3.
Autophagy is a lysosomal degradation pathway and serves an adaptive role in diverse pathologies. Currently, the role of autophagy in kidney disease remains controversial, as it is involved in both cell survival and death depending on different stimuli or cellular environment.12, 52 Our study provides in vitro and in vivo evidence that sustained HG resulted in defective autophagy in glomerulus and podocyte, which could be partially corrected by UDCA or 4-PBA treatment.
An interesting discovery in this study is that treatment with UDCA or 4-PBA not only decreased blood glucose level but also lowered systolic blood pressure. Mice with constitutive mutations in ER stress proteins develop diabetes, defects in glucose handling and hypertension.53, 54 Inhibition of ER stress in male s.d. rats given Ang II and treated with either tauroursodeoxycholic acid (TUDCA) or PBA led to a 20 mm Hg decrease in blood pressure with either inhibitor, compared with Ang II treatment alone.55 The Ang II-treated rats receiving either TUDCA or PBA had normalized levels of serum creatinine, a characteristic of impaired renal function, suggesting an attenuation of renal dysfunction in these rats.56 In our study, both UDCA and 4-PBA improved renal dysfunction and decreased blood pressure after treatment for 8 weeks. It remains unclear to which part the amelioration of DN is a direct effect of blood glucose and pressure lowering. At least, the reduced albuminuria is related to ER stress-induced apoptosis in podocyte.
In conclusion, hyperglycemia-induced podocyte apoptosis through ER stress contributes to the pathogenesis of DN in diabetic db/db mice. Both UDCA and 4-PBA prevent the development of DN in db/db mice by improving glucose or insulin tolerance, reversing mesangial expansion, regulating autophagy and inhibiting apoptosis. Moreover, ER stress is involved in HG-induced apoptosis in podocytes in vitro. Both UDCA and 4-PBA prevent HG-induced podocyte apoptosis by alleviating ER stress and restoring autophagy. Therefore, UDCA and 4-PBA are potentially attractive agents for the treatment of DN.
References
Xu Y, Wang L, He J et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959.
Yang W, Lu J, Weng J et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–1101.
Zhang L, Wang F, Wang L et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–822.
van Dijk PR, Kramer A, Logtenberg SJ et al. Incidence of renal replacement therapy for diabetic nephropathy in the Netherlands: Dutch diabetes estimates (DUDE)-3. BMJ Open 2015;5:e005624.
Kang AY, Park SK, Park SY et al. Therapeutic target achievement in type 2 diabetic patients after hyperglycemia, hypertension, dyslipidemia management. Diabetes Metab J 2011;35:264–272.
Ito N, Nishibori Y, Ito Y et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab Invest 2011;91:1584–1595.
Chen Y, Gui D, Chen J et al. Down-regulation of PERK-ATF4-CHOP pathway by Astragaloside IV is associated with the inhibition of endoplasmic reticulum stress-induced podocyte apoptosis in diabetic rats. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 2014;33:1975–1987.
Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529.
Liu G, Sun Y, Li Z et al. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 2008;370:651–656.
Cunard R, Sharma K . The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol 2011;300:F1054–F1061.
Lindenmeyer MT, Rastaldi MP, Ikehata M et al. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 2008;19:2225–2236.
Fang L, Zhou Y, Cao H et al. Autophagy attenuates diabetic glomerular damage through protection of hyperglycemia-induced podocyte injury. PLoS One 2013;8:e60546.
Hartleben B, Godel M, Meyer-Schwesinger C et al. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J Clin Invest 2010;120:1084–1096.
Meyer TW, Bennett PH, Nelson RG . Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 1999;42:1341–1344.
Wolf G, Chen S, Ziyadeh FN . From the periphery of the glomerular capillary wall toward the center of disease: podocyte injury comes of age in diabetic nephropathy. Diabetes 2005;54:1626–1634.
Eid AA, Gorin Y, Fagg BM et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes 2009;58:1201–1211.
Festi D, Montagnani M, Azzaroli F et al. Clinical efficacy and effectiveness of ursodeoxycholic acid in cholestatic liver diseases. Curr Clin Pharmacol 2007;2:155–177.
Woo SJ, Kim JH, Yu HG . Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model. J Ocul Pharmacol Ther 2010;26:223–229.
Boatright JH, Moring AG, McElroy C et al. Tool from ancient pharmacopoeia prevents vision loss. Mol Vis 2006;12:1706–1714.
Ozcan U, Yilmaz E, Ozcan L et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006;313:1137–1140.
Carlisle RE, Brimble E, Werner KE et al. 4-Phenylbutyrate inhibits tunicamycin-induced acute kidney injury via CHOP/GADD153 repression. PLoS One 2014;9:e84663.
Chan JY, Luzuriaga J, Bensellam M et al. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in beta-cell gene expression and progression to diabetes. Diabetes 2013;62:1557–1568.
Siegel AS, Henley S, Zimmerman A et al. The influence of passive stretch and NF-kappaB inhibitors on the morphology of dystrophic muscle fibers. Anat Rec (Hoboken) 2011;294:132–144.
Lupachyk S, Watcho P, Stavniichuk R et al. Endoplasmic reticulum stress plays a key role in the pathogenesis of diabetic peripheral neuropathy. Diabetes 2013;62:944–952.
Wang P, Yan Z, Zhong J et al. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012;61:2155–2165.
Zhang H, Zhao T, Gong Y et al. Attenuation of diabetic nephropathy by Chaihuang-Yishen granule through anti-inflammatory mechanism in streptozotocin-induced rat model of diabetics. J Ethnopharmacol 2014;151:556–564.
Taneda S, Pippin JW, Sage EH et al. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol 2003;14:968–980.
Atmaca A, Al-Batran SE, Maurer A et al. Valproic acid (VPA) in patients with refractory advanced cancer: a dose escalating phase I clinical trial. Br J Cancer 2007;97:177–182.
Mundel P, Reiser J, Zuniga Mejia Borja A et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 1997;236:248–258.
Cao A, Li Q, Yin P et al. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis 2013;18:1391–1402.
Susztak K, Raff AC, Schiffer M et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 2006;55:225–233.
Xue W, Lei J, Li X et al. Trigonella foenum graecum seed extract protects kidney function and morphology in diabetic rats via its antioxidant activity. Nutr Res 2011;31:555–562.
Pan D, Zhang D, Wu J et al. A novel proteoglycan from Ganoderma lucidum fruiting bodies protects kidney function and ameliorates diabetic nephropathy via its antioxidant activity in C57BL/6 db/db mice. Food Chem Toxicol 2014;63:111–118.
Zhang Z, Li BY, Li XL et al. Proteomic analysis of kidney and protective effects of grape seed procyanidin B2 in db/db mice indicate MFG-E8 as a key molecule in the development of diabetic nephropathy. Biochim Biophys Acta 2013;1832:805–816.
Tesch GH, Lim AK . Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy. Am J Physiol Renal Physiol 2011;300:F301–F310.
Sharma K, McCue P, Dunn SR . Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 2003;284:F1138–F1144.
Neschen S, Scheerer M, Seelig A et al. Metformin supports the antidiabetic effect of a sodium glucose cotransporter 2 inhibitor by suppressing endogenous glucose production in diabetic mice. Diabetes 2015;64:284–290.
Coward RJ, Welsh GI, Yang J et al. The human glomerular podocyte is a novel target for insulin action. Diabetes 2005;54:3095–3102.
Welsh GI, Hale LJ, Eremina V et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab 2010;12:329–340.
Lennon R, Pons D, Sabin MA et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant 2009;24:3288–3296.
van Belle TL, Coppieters KT, von Herrath MG . Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 2011;91:79–118.
Lee SC, Pervaiz S . Apoptosis in the pathophysiology of diabetes mellitus. Int J Biochem Cell Biol 2007;39:497–504.
Cao Y, Hao Y, Li H et al. Role of endoplasmic reticulum stress in apoptosis of differentiated mouse podocytes induced by high glucose. Int J Mol Med 2014;33:809–816.
Inagi R, Nangaku M, Onogi H et al. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int 2005;68:2639–2650.
Nakatani Y, Kaneto H, Kawamori D et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem 2005;280:847–851.
Ozawa K, Miyazaki M, Matsuhisa M et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 2005;54:657–663.
Bek MF, Bayer M, Muller B et al. Expression and function of C/EBP homology protein (GADD153) in podocytes. Am J Pathol 2006;168:20–32.
Maris M, Overbergh L, Gysemans C et al. Deletion of C/EBP homologous protein (Chop) in C57Bl/6 mice dissociates obesity from insulin resistance. Diabetologia 2012;55:1167–1178.
Wu J, Zhang R, Torreggiani M et al. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 2010;176:2163–2176.
Nakagawa T, Yuan J . Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 2000;150:887–894.
Nakagawa T, Zhu H, Morishima N et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000;403:98–103.
Banerjee P, Basu A, Wegiel B et al. Heme oxygenase-1 promotes survival of renal cancer cells through modulation of apoptosis- and autophagy-regulating molecules. J Biol Chem 2012;287:32113–32123.
Zhang P, McGrath B, Li S et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 2002;22:3864–3874.
Young CN, Li A, Dong FN et al. Endoplasmic reticulum and oxidant stress mediate nuclear factor-kappaB activation in the subfornical organ during angiotensin II hypertension. Am J Physiol Cell Physiol 2015;308:C803–C812.
Chao YM, Lai MD, Chan JY . Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension 2013;61:1270–1280.
Spitler KM, Webb RC . Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension 2014;63:e40–e45.
Acknowledgements
This study was supported by the Innovation Program of Shanghai Municipal Education Commission (14ZZ118); the Leading Academic Discipline Project of State Administration of Traditional Chinese Medicine of China, the Talent Project of Integrative Medicine of Shanghai Municipal Health Bureau (ZYSNXD012-RC-ZXY); and the National Natural Science Foundation of China (81400728 and 81473480).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This paper describes the effects of the ER stress inhibitors ursodeoxycholic acid (UDCA) and 4-phenylbutyrate (4-PBA) on diabetic nephropathy (DN) in mice. UDCA and 4-PBA prevent hyperglycemia-induced podocyte apoptosis stress and restorine autophagy, decrease urinary albuminuria, attenuate mesangial expansion and prevent apoptosis in podocytes.
Rights and permissions
About this article
Cite this article
Cao, AL., Wang, L., Chen, X. et al. Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest 96, 610–622 (2016). https://doi.org/10.1038/labinvest.2016.44
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.44
This article is cited by
-
Vitamin D receptor attenuate ischemia-reperfusion kidney injury via inhibiting ATF4
Cell Death Discovery (2023)
-
Endoplasmic reticulum stress in diabetic kidney disease: adaptation and apoptosis after three UPR pathways
Apoptosis (2023)
-
Mutation of Beclin1 acetylation site at K414 alleviates high glucose-induced podocyte impairment in the early stage of diabetic nephropathy by inhibiting hyperactivated autophagy
Molecular Biology Reports (2022)
-
Modes of podocyte death in diabetic kidney disease: an update
Journal of Nephrology (2022)
-
Naringenin alleviates hyperglycemia-induced renal toxicity by regulating activating transcription factor 4–C/EBP homologous protein mediated apoptosis
Journal of Cell Communication and Signaling (2022)