Abstract
Renal ischemia–reperfusion (I/R) injury is unavoidable in kidney transplantation (KTx) and frequently influences both short- and long-term allograft survival. Carbon monoxide (CO) has attracted attention as a medical gas with anti-inflammatory and anti-apoptotic effects. We investigated a new strategy for organ preservation using ex vivo application of high-pressure CO in an experimental rat KTx model. We preserved kidney grafts using a high-pressure chamber filled with mixed gases composed of CO and O2. We found that cold I/R injury resulted in progressive deterioration of renal graft function in University of Wisconsin solution, whereas CO significantly improved renal function. We confirmed that CO decreased oxidative stress and mRNA expression of proinflammatory cytokines and inhibited tubular apoptosis in the early phases. Western blot analysis demonstrated that CO increased phosphatidylinositol-3 kinase and phosphorylation of Akt and p38 mitogen-activated protein kinase. Furthermore, CO significantly alleviated tubular injury scores and suppressed the development of interstitial fibrosis at 100 days after KTx. Thus, high-pressure mixed CO and O2 gases successfully preserved rat kidney grafts for 24 h by protecting tubular epithelial cells from apoptosis and inhibiting inflammation.
Similar content being viewed by others
Main
Ischemia–reperfusion (I/R) injury has a major role in delayed graft function and long-term changes after kidney transplantation.1 Furthermore, cold ischemic time is a potential risk factor for graft survival in recipients of kidneys from cardiac-death donors.2 A cold ischemia time of less than 12 h is strongly associated with superior graft survival, but few kidneys from cardiac-death donors have such a short cold ischemic time.3 Demand for kidney transplantation (KTx) far exceeds the supply of donor organs, and the shortfall is becoming more severe as donor numbers fail to keep pace with the increasing numbers of patients listed for transplantation.4 The current shortage of deceased-donor kidney grafts means that surgeons are now transplanting organs that would previously have been considered unacceptable, such as those from non-heart-beating donors. Today, there is an increasing need for solutions that afford improved protection to organs from marginal donors. Thus, new strategies to increase graft protection during cold preservation are urgently needed.5
Recently, heme oxygenase (HO)-derived carbon monoxide (CO) has emerged. CO exerts vasoactive, anti-proliferative, antioxidant, anti-inflammatory, and anti-apoptotic effects and contributes substantially to the important role of the inducible HO-1 isoform as a mediator of tissue protection and host defense.6 Exogenous application of low doses of gaseous CO might provide a powerful tool to protect organs and tissues under various stressful conditions. Experimental evidence suggests a beneficial effect of gaseous CO under pathophysiological conditions, such as organ transplantation, I/R, inflammation, sepsis, or shock states.6 Hatayama et al performed a cervical ectopic heart transplantation after preserving a rat heart for 48 h using a high-pressure chamber filled with a mixed gas of CO (PCO=4000 hPa) and O2 (PO2=3000 hPa) and confirmed resuscitation of the transplanted heart.7 The present study assumed that rat kidney grafts could be preserved under desiccating conditions with CO and O2 at 4 °C for 24 h. CO protected tubular epithelial cells from apoptosis and inflammation, and thereby reduced cold I/R injury.
MATERIALS AND METHODS
Animals
Inbred male Lewis (LEW) rats, weighing 250–350 g, were purchased from SLC Japan (Hamamatsu, Japan). The animals were maintained in a controlled temperature and light environment and allowed free access to a standard diet and water throughout the experimental periods.
All studies were performed in accordance with the principles of the guidelines for animal experimentation at Osaka University and the National Research Institute for Child Health and Development.
Kidney Transplantation
Orthotopic KTx was performed using a technique described previously.8, 9 Donor male LEW rats were anesthetized with 50 mg/kg pentobarbital sodium (Nembutal, Abbott Laboratories, Chicago, IL, USA) and heparinized with 500 U/body heparin before surgery. Anesthetized animals were subjected to a midline laparotomy. The left kidney was flushed with ice-cold University of Wisconsin (UW) solution (Viaspan, DuPont, Wilmington, DE, USA) via the abdominal aorta and was removed. The excised graft was preserved in UW solution or in a high-pressure chamber filled with mixed gas at 4 °C for 24 h. After a left nephrectomy on a similarly anesthetized recipient LEW rat, the kidney graft was transplanted orthotopically into the recipient by end-to-end anastomosis of the left renal vessels and ureter with 10-0 sutures using microsurgical techniques. The remaining right native kidney was removed 10 days after KTx for experiments to assess renal function.
Ex Vivo Application of Mixed High-Pressure Gas during Cold Preservation
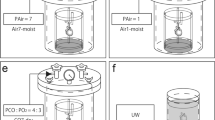
The isolated kidney was placed in a high-pressure chamber without preservation solution, which was cooled beforehand to 4 °C (refs 7, 10, 11; Figure 1a). Subsequently, the high-pressure chamber was filled with mixed gas composed of CO (PCO=2000 hPa) and O2 (PO2=1500 hPa) or N2 (PN2=2000 hPa) and O2 (PO2=1500 hPa). After preservation at 4 °C for 24 h, the kidney graft was removed from the chamber, and an orthotopic KTx was performed.
Illustration of a high-pressure chamber and the experimental protocols. (a) A hyperbaric chamber was used to preserve the isolated kidneys. A beaker containing distilled water (DW) was placed in the chamber to maintain the humidity inside the chamber at >90%. The kidney was flushed with University of Wisconsin (UW) solution and placed as shown in the illustration. A mixture of CO (or N2) and O2 was pressurized to 3500 hPa, and the chamber was put in a refrigerator maintained at 4 °C for 24 h. (b) Experimental protocols. Four groups of animals were examined: recipients with non-preserved grafts (control group), recipients with grafts preserved in UW solution for 24 h (UW group), and recipients with grafts preserved for 24 h in a high-pressure chamber containing CO or N2 (CO or N2 group). The blood and urine samples were collected at 30, 60, and 90 days after kidney transplantation. Recipients were killed to obtain graft kidney samples at 3 and 24 h, or 100 days after kidney transplantation.
Experimental Protocols
Syngeneic KTx was performed, and four groups of animals were examined: recipients with non-preserved grafts (control group), recipients with grafts preserved in UW solution for 24 h (UW group), and recipients with grafts preserved for 24 h in a high-pressure chamber containing CO or N2 (CO or N2 group; Figure 1b). Blood and urine were obtained at 30, 60, and 90 days after KTx. Recipients were killed at 3, 24 h, and 100 days after KTx, and the kidneys were removed after perfusion with 20 ml of ice-cold PBS. The cortex was carefully dissected from the medulla and then processed for evaluation by mRNA and protein analysis. Tissues for mRNA and protein extraction were frozen using liquid nitrogen, and tissues for light microscopy were fixed with 4% paraformaldehyde (PFA) overnight, dehydrated through a graded ethanol series, and embedded in paraffin.
Assessment of Carboxyhemoglobin (CO-Hb) by Blood Gas Analysis
Arterial blood was obtained from naive LEW rats and CO-treated recipients at 3 and 24 h after KTx. CO-Hb was analyzed with a blood gas analyzer (ABL735, Radiometer, Brønshøj, Denmark).
Renal Function
Serum creatinine (Cr) and blood urea nitrogen (BUN) levels were measured by the creatinase-peroxidase method and the urease-ultraviolet method, respectively (SRL, Tokyo, Japan). Protein and Cr levels in 24 h urine samples were determined using a metabolic cage system. The formula for creatinine clearance is: (CCr; ml/min)=(U [Cr] × urine volume)/(S [Cr] × time), where U [Cr] is urinary creatinine (mg/dl), urine volume is in milliliters, and S [Cr] is serum creatinine (mg/dl).
Morphological Analysis
Tissue samples were fixed in 4% PFA for 24 h and embedded in paraffin. Then, 3-μm tissue sections were mounted on saline (2% 3-aminopropyltriethoxysilane)-coated slides and deparaffinized with xylene. Histological sections were stained with periodic acid-Schiff (PAS) and Masson’s trichrome stains. Tubular injury was scored by estimating the percentage of tubules in the outer medulla and corticomedullary junction that showed epithelial necrosis or had necrotic debris or cast as follows: 0, none; 1+, <10%; 2+, 10–25%; 3+, 26–45%; 4+, 46–75%; 5+, >75%.12 Ten viewing fields randomly selected from the outer medulla and corticomedullary junction on each slide section were examined at × 200 magnification. The interstitial fibrotic area was stained blue with Masson’s trichrome staining, and a color image analyzer (WinROOF, ver. 5.5, MITANI, Tokyo, Japan) estimated the area quantitatively. All histological slides were examined by light microscopy using a Nikon Eclipse 80i (Nikon, Tokyo, Japan); pictures were taken with Nikon ACT-1 (ver. 2.63). The scores of 10 fields per kidney were averaged, and the mean scores for each group were averaged.
TUNEL Staining
Terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) staining was performed using the in situ Apoptosis Detection Kit (Takara Bio, Ohtsu, Japan), according to the manufacturer’s instructions. Briefly, the sections were deparaffinized and treated with proteinase K (10 μg/ml, Takara Bio) for 15 min at room temperature. The slides were then incubated with peroxidase blocking solution (Dako, Hamburg, Germany) for 10 min, followed by incubation in TdT enzyme solution for 60 min at 37 °C. The reaction was terminated by incubating in a stop/wash buffer for 30 min at 37 °C. Chromogenic color was developed with DAB (Dako), and the nuclei were counterstained with hematoxylin. The number of TUNEL-positive cell nuclei was counted in 10 random high-power fields (× 200) of each slide and averaged.
Antibodies and Western Blot Analysis
The following antibodies were used for immunochemical testing to detect the pathways protecting the kidneys: polyclonal phosphatidylinositol-3 kinase (PI3K) p85a antibody, polyclonal phospho-Akt (Ser473) antibody, polyclonal phospho-p38 mitogen-actiated protein kinase (MAPK; Thr180/Tyr182) antibody, polyclonal phospho-p44/42 MAPK (Erk1/2; Thr202/Tyr204) antibody, and polyclonal phospho-stress-activated protein kinase (SAPK)/c-jun N-terminal kinase (JNK; Thr183/Tyr185) antibody (1:10 000, Cell Signaling Technology, Beverly, MA, USA). Protein levels were normalized with polyclonal β-actin antibody (1:10 000, Cell Signaling Technology).
Kidney tissue was homogenized in radioimmunoprecipitation lysis buffer with phenylmethylsulfonylfluoride solution, sodium orthovanadate solution, protease inhibitor (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and phosphatase inhibitor (Sigma-Aldrich, St Louis, MO, USA). Homogenates were centrifuged (12 000 g, 10 min, 4 °C), and the supernatant total protein was measured using the Lowry protein assay (Bio-Rad Laboratories, Hercules, CA, USA). Total protein lysates (15 μg), containing 1:1 denaturing sample buffer, were boiled for 3 min, resolved on 10% SDS–polyacrylamide gels, and electrophoretically transferred to a PVDF membrane (Bio-Rad). The filter was blocked with 2% ECL Advance Blocking Agent (Amersham Bioscience, Piscataway, NJ, USA) in 10 mM Tris-buffered saline with 0.1% Tween 20 (TBS-T), followed by an overnight incubation at 4 °C with diluted primary antibodies in blocking buffer. After washing six times in TBS-T, the filter was incubated with secondary antibody (1:10 000; Cell Signaling Technology) in blocking buffer for 60 min at room temperature and developed to detect specific protein bands using ChemiDocXRS (Bio-Rad) and ECL Advance Reagents (Amersham Bioscience).
RNA Isolation and Quantitative Real-Time RT-PCR
Total RNA was extracted from kidney grafts using an RNeasy Mini Kit (QIAGEN, Valencia, CA, USA), according to the manufacturer’s protocol. Total RNA was determined by measuring the optical density at 260 nm. Each 600 ng of RNA was reverse transcribed to cDNA using oligo(dT) primers and SuperScript reverse transcriptase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. Quantitative RT-PCR was performed using the TaqMan program on an Applied Biosystems 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Primers amplifying the rat mRNA regions and a specific Taqman probe were designed using the Primer Express software package (Applied Biosystems). Data are expressed as the comparative cycle threshold (Ct). The normalized Ct value of each gene was obtained by subtracting the Ct value of 18s rRNA. The fold change vs one sample of the control group was calculated as described previously.13
Oxidative Damage Measurement
The oxidative stress marker 8-hydroxydeoxyguanosine (8-OHdG) was measured with an enzyme-linked immunosorbent assay kit (Japan Institute for the Control of Aging, Shizuoka, Japan), according to the manufacturer’s instructions. 8-OHdG, a product of oxidatively damaged DNA, was detected using a competitive enzyme-linked immunosorbent assay using a monoclonal antibody (clone N45.1) that is specific for DNA damage.
Statistical Analysis
Data are expressed as means±s.d. The Kaplan–Meier method was used to calculate survival rates after KTx. Log-rank tests were used for comparisons between the two groups. Statistical analysis was performed using Fisher’s LSD test and Tukey test for parametric multiple comparisons. Differences were considered statistically significant at P<0.05.
RESULTS
Recipient Survival Rates
Figure 2a and b shows the recipient survival rates assessed at 100 days after KTx. We performed an orthotopic KTx after preserving a rat kidney for 24 h using a high-pressure chamber filled with a mixed gas of CO (PCO=4000 hPa) and O2 (PO2=3000 hPa) according to the previous study in a cervical ectopic heart transplantation model.7 However, no protective effect of high-pressure CO (PCO=4000 hPa) and O2 (PO2=3000 hPa) was observed compared with the group preserving a rat kidney for 24 h in a chamber that was filled with room air (1000 hPa). Then, we selected a lower mixed gas composed of CO (PCO=2000 hPa) and O2 (PO2=1500 hPa) for kidney graft, resulting in improved graft survival (Figure 2a). Next, four groups of animals were examined: recipients with non-preserved grafts (control group), recipients with grafts preserved in UW solution for 24 h (UW group), and recipients with grafts preserved for 24 h in a high-pressure chamber containing CO (PCO=2000 hPa, CO group) and O2 (PO2=1500 hPa), or N2 (PN2=2000 hPa, N2 group) and O2 (PO2=1500 hPa). No significant difference was observed among the non-preserved control group (100%, n=10), UW group (78.6%, n=14), CO group (84.6%, n=13), or N2 group (71.1%, n=9).
Recipient survival rates and percent carboxyhemoglobin (CO-Hb). Recipient survival rates were assessed by following animals for 100 days after transplantation. (a) An orthotopic KTx was performed after preserving a rat kidney for 24 h using a chamber filled with a mixed gas of CO (PCO=4000 hPa) and O2 (PO2=3000 hPa; n=4), CO (PCO=2000 hPa) and O2 (PO2=1500 hPa; n=14), or room air (1000 hPa; n=5). (b) No significant differences were observed among the control (n=10), UW (n=14), CO (PCO=2000 hPa+PO2=1500 hPa, n=13), and N2 (PN2=2000 hPa+PO2=1500 hPa, n=9) groups. (c) Percent CO-Hb in the blood of naive Lewis rats and recipients with CO-treated grafts at 3 and 24 h after kidney transplantation (n=3/group). Fisher's LSD test, *P<0.05.
Effect of Carbon Monoxide on CO-Hb
The ex vivo application of high-pressure CO significantly increased recipient CO-Hb levels to 1.10±0.72% at 3 h after KTx compared with naive LEW rats (0.03±0.06%, P<0.05; Figure 2c). At 24 h after KTx, CO-Hb levels returned to 0.10±0.10% (P<0.05 vs 3 h after KTx).
Effect of Carbon Monoxide on Renal Function
Figure 3 summarizes the effects of CO on renal function in the rat KTx models. Although no significant difference was observed in survival rates among the experimental groups, cold I/R injury resulted in progressive deterioration of renal graft function. Treatment with CO, but not N2, significantly suppressed the increase in Cr and BUN at 30, 60, and 90 days after KTx (Figure 3a and b). CCr significantly decreased to 1.90±0.53 ml/min in the UW-preserved group at 60 days after KTx (vs control group, 2.86±0.44 ml/min; P<0.01; Figure 3c). In contrast, treatment with CO maintained the CCr level (2.27±0.65 ml/min; not significant vs control group), while N2 failed (1.12±0.77 ml/min; P<0.01 vs CO group). Furthermore, recipients in the UW group exhibited significant proteinuria (55.3±17.1 mg per 24 h) at 60 days after KTx, compared with control rats (14.3±2.5 mg per 24 h; Figure 3d). Recipients in the CO group showed significantly lower protein excretion (24.5±11.2 mg per 24 h; P<0.01 vs UW group), whereas those in the N2 group did not (50.9±26.0 mg per 24 h; not significant vs UW group).
Carbon monoxide protects renal function. Renal graft function was assessed by serum creatinine (a), blood urea nitrogen (b), creatinine clearance (CCr) (c), and 24 h urine protein (d) at 30, 60, and 90 days after kidney transplantation (KTx). The syngenic KTx procedure without cold preservation did not affect graft function, whereas prolonged cold ischemia for 24 h resulted in a decrease in CCr with considerable proteinuria. The ex vivo application of high-pressure CO significantly improved graft function compared with the UW and N2 groups. Tukey’s test, **P<0.01, *P<0.05.
Effect of Carbon Monoxide on Tubular Injury and Interstitial Fibrosis
PAS staining revealed that cold I/R injury induced characteristic histological changes, including tubular atrophy and dilation, loss of the brush border, inflammatory cell infiltration, and cast formation at 100 days after KTx (Figure 4a). However, CO treatment significantly alleviated tubular injury scores compared with the UW and N2 groups (Figure 4b). The interstitial fibrotic area was stained blue with Masson’s trichrome staining (Figure 4c), and a color image analyzer was used to estimate the area quantitatively (Figure 4d). The UW and N2 groups showed a progression of interstitial fibrosis. In parallel with the PAS staining findings, CO treatment significantly ameliorated the development of interstitial fibrosis.
Renal histopathological findings. Representative photomicrographs show the renal morphological changes seen on periodic acid-Schiff (PAS) staining (a) and Masson’s trichrome staining (c) at 100 days after kidney transplantation. Quantitative analysis of tubular injury with PAS staining (b) and interstitial fibrosis with Masson’s trichrome staining (d) are summarized. The data are expressed as means±s.d. Tukey’s test, **P<0.01, *P<0.05.
Morphological Evidence of Apoptosis
To investigate whether CO treatment could protect tubular cells from apoptosis, the apoptotic bodies, labeled as an in situ end-labeled DNA fragment with the TUNEL method, were examined during the early phases (Figure 5). Tubular apoptosis increased persistently after cold I/R injury in the UW-preserved kidneys (14.4±4.8, 24.1±4.0, and 14.2±5.0 cells per high-power field, at the end of cold storage, 3 and 24 h after KTx, respectively). Treatment with CO significantly repressed tubular apoptosis (1.5±0.6, 4.8±2.1, and 4.4±2.7 cells per high-power field, at the end of cold storage, 3 and 24 h after KTx, respectively), whereas N2 did not (14.7±6.5, 22.5±0.6, and 21.5±2.2 cells per high-power field, at the end of cold storage, 3 and 24 h after KTx, respectively).
Carbon monoxide decreases tubular apoptosis. Many TUNEL-positive cells were detected in the outer medulla following prolonged cold ischemia for 24 h and were suppressed by CO treatment at the end of cold storage (a), 3 (c) and 24 h after kidney transplantation (e). TUNEL-positive cells (b, d, f) were counted in tubular cells of the outer medulla at × 200 magnification in a minimum of 10 fields (n=3/group). Data are expressed as means±s.d. Tukey’s test, **P<0.01, *P<0.05.
Effect of Carbon Monoxide on Cell Signaling
Cortical expression of the PI3K–Akt and MAPK pathways was examined to elucidate the intracellular signaling implicated in tubular protection (Figure 6). A western blot analysis demonstrated that treatment with CO increased PI3K and Akt phosphorylation in the early phases after KTx. Furthermore, CO selectively activated p38 MAPK but had no effect on ERK1/ERK2 or JNK MAP kinases.
Effect of carbon monoxide on cell signaling. To examine the intracellular CO signaling implicated in tubular protection, the expression of PI3K and the activation of Akt and MAPK were examined at 3 (a) and 24 h after kidney transplantation (b). Western blot analysis demonstrated that treatment with CO increased PI3K and Akt and p38 MAPK phosphorylation.
Cortical Tissue mRNA Expression
As shown in Figure 7, a real-time RT-PCR analysis demonstrated that CO treatment decreased interferon (IFN)-γ at 3 h after KTx (P<0.05) and inducible nitric oxide synthase (iNOS) mRNA levels at 24 h after KTx (P<0.01) compared with simple cold storage using UW solution. We also assessed activation of the heme-degrading enzyme HO-1, which has a primary role in eliminating toxic free heme and protects cells from heme-induced oxidative stress.14 HO-1 mRNA levels in the kidney grafts were elevated 24 h after KTx in the UW group, whereas the mixed gas treatment significantly reduced HO-1 mRNA levels (P<0.01).
Cortical mRNA expression by real-time RT-PCR assay. The relative mRNA expression of interferon (IFN)-γ, inducible nitric oxide synthase (iNOS), interleukin (IL)-6, and heme oxygenase-1 (HO-1) at 3 (a) and 24 h after kidney transplantation (b) is shown. Changes in mRNA expression vs one sample of the control group were calculated. The relative quantity is presented as the ratio of the comparative cycle threshold (Ct) of the target genes against those of housekeeping gene 18. Data are representative of three independent experiments and indicate the mean ratio of triplicate results from each experiment. Data are expressed as means±s.d. Tukey’s test, **P<0.01, *P<0.05.
Effects of Carbon Monoxide on Oxidative Stress
Serum levels of 8-OHdG were examined to evaluate the effects of CO on oxidative stress (Figure 8). Prolonged cold ischemia for 24 h resulted in an increase in serum 8-OHdG levels at 3 h after KTx (0.22±0.05 ng/ml in the control group, 0.32±0.10 ng/ml in the UW group, and 0.29±0.04 ng/ml in the N2 group), whereas CO treatment ameliorated oxidative DNA injury (0.22±0.03 ng/ml).
Carbon monoxide ameliorates serum levels of 8-hydroxydeoxyguanosine (8-OHdG). Serum levels of 8-OHdG, an oxidative stress marker, at 3 h after kidney transplantation are shown. Prolonged cold ischemia for 24 h resulted in an increase in 8-OHdG serum levels, whereas CO treatment ameliorated oxidative DNA injury. Data are expressed as means±s.d (n=3/group).
DISCUSSION
We developed a new strategy to preserve organs using high-pressure CO. Previous studies have reported successful organ preservation using a high-pressure chamber filled with a mixed gas in a cervical ectopic heart transplantation model.7, 10, 11 Here, we evaluated long-term graft function after preserving the graft using this method in a rat kidney transplantation model as a life-supporting model.
CO has anti-inflammatory and anti-apoptotic effects and has attracted attention as a medical gas.15 Zhang et al16 demonstrated that the anti-apoptotic effects of CO involve both the PI3K/Akt and p38 MAPK signaling pathways in endothelial cells in an anoxia-reoxygenation injury model. Nakao and colleagues also demonstrated that low-concentration CO inhalation provided protection against cold I/R injury in a rat kidney transplantation model.17 Similar protective results can be achieved after storage of grafts in a UW solution saturated with CO.18, 19 Sener et al20 demonstrated that carbon monoxide releasing molecules (CORM) supplementation in UW solution has a significant impact on decreasing cellular and graft injury, and improving rat kidney graft survival through its anti-apoptotic effects. Recently, Ruan Y et al21 reported the protective effect of CO from CORM on renal ischemia–reperfusion injury is associated with the inhibition of high-mobility group box 1 (HMGB1) translocation and release. In addition to its anti-inflammatory and anti-apoptotic effects, CO reduced graft immunogenicity and inhibited chronic allograft nephropathy in a rat kidney transplantation model.22 Although CO may cause serious adverse effects, these results indicate that CO is a possible therapeutic tool for deceased-donor organ transplantation.
Cold I/R injury resulted in progressive deterioration of renal graft function, whereas ex vivo application of high-pressure CO gas significantly improved renal function compared with simple cold storage using UW solution (Figure 3). This finding was confirmed by our observation that replacing CO with N2 resulted in a loss of the protective effect. Histopathologically, CO treatment significantly alleviated tubular injury scores and suppressed the development of interstitial fibrosis compared with the UW and N2 groups at 100 days after KTx (Figure 4).
We demonstrated that during the early phases, CO treatment decreased oxidative stress and mRNA expression of proinflammatory cytokines, thereby inhibiting tubular apoptosis. CO-mediated activation of p38 MAPK exerts an anti-inflammatory effect.23 In the present study, CO selectively activated p38 MAPK but had no effect on ERK1/ERK2 or JNK MAP kinases (Figure 6). Consistent with these findings, we also found that CO treatment decreased IFN-γ and iNOS mRNA levels after cold I/R injury. Furthermore, our data show that tubular apoptosis was stimulated during cold ischemia and after reperfusion, whereas CO significantly inhibited tubular apoptosis (Figure 5). We demonstrated that CO treatment increased PI3K, and, induced Akt phosphorylation after cold I/R injury. Several downstream targets of Akt (such as E2F, c-myc, Bad) have been implicated in cell cycle progression and anti-apoptosis.24 Activation of Akt protects a wide variety of cells from apoptosis. Thus, our results indicate that the suppressive mechanism of tubulointerstitial injury may be associated with the anti-apoptotic effect, through the PI3K–Akt pathway. One of the major events in I/R-induced kidney injury is the generation of cytotoxic oxygen radicals.25 An increase in cytotoxic oxygen radicals leads to increased cellular injury, including DNA damage, protein oxidation and nitrosylation, lipid peroxidation, and apoptosis.26 We examined serum 8-OHdG levels to evaluate the effects of CO on oxidative stress (Figure 8). Prolonged cold ischemia for 24 h resulted in an increase in serum 8-OHdG levels at 3 h after KTx, whereas CO treatment ameliorated oxidative DNA injury, suggesting that CO treatment during cold storage reduced injurious reactive oxygen species (ROS). Several possible mechanisms have been postulated to explain the antioxidant effects of CO. Nakao et al18 demonstrated that CO in the organ preservation solution used during transplantation binds to and stabilizes renal cytochrome P450 (CYP) and prevents CYP degradation and detrimental heme/iron release in renal grafts. Brugger et al27 demonstrated that the antioxidant effects afforded by CO involve activating the p38 MAPK pathway. In this study, it was unclear whether CO suppressed oxidative stress directly or indirectly, but applying CO resulted in suppression of oxidative stress and inhibition of inflammation and apoptosis. Furthermore, several recent studies have shown that CO mediates cytoprotection through the induction of HO-1.28, 29 However, in this study, endogenous HO-1 was upregulated in kidney grafts preserved with UW solution, whereas CO-treated kidneys showed less HO-1 expression. These results suggest that endogenous HO-1 expression is associated with renal graft injury, and that the cytoprotective effect of CO does not depend on HO-1 activation.
Oxygenation of the preservation solution is mandatory during hypothermic machine-perfusion of abdominal organs. Hart et al30 demonstrated better results with 21% and 95% oxygenation, compared with preserving a rat liver without oxygenation in a UW machine-perfusion solution. In addition, they showed that toxic ROS are generated not only during warm incubation but also during cold preservation, and that 21% oxygen saturation produced optimal results. With the aim of achieving organ preservation under better conditions, we attempted to adjust the optimal gas partial pressure and selected a mixed gas composed of PCO=2000 hPa+PO2=1500 hPa for kidney graft preservation.
However, CO binds hemoglobin with an affinity that is 240 times greater than that of oxygen, thereby interfering with the oxygen delivery system of the body. CO-Hb levels correlate well with clinical symptoms of CO poisoning in humans; levels of 10–30% can cause minor transient symptoms including headache, dizziness, and shortness of breath; death occurs at CO-Hb levels between 50 and 80%.31 Our study revealed that ex vivo application of high-pressure CO slightly increased CO-Hb levels at 3 h after KTx, to 1.10±0.72%, but levels then dropped to naive LEW rat levels by 24 h after KTx (Figure 2b), suggesting that our procedure may be clinically acceptable. The safety of our method using high-pressure CO for clinicians and transporters is also confirmed.32 When filling the gases into the chamber, CO gas was not detected directly above the chamber. When opening the valve of the chamber inside the fume hood, CO gas was detected on average 260 p.p.m. at approximately 60 s, decreased at approximately 180 s, and then was barely detectable at approximately 240 s. Meanwhile, CO gas was not detected at any time outside the fume hood.
In conclusion, we successfully preserved rat kidney grafts for 24 h under high-pressure CO by protecting tubular epithelial cells from apoptosis and inhibiting inflammation. Our new method of organ preservation is a groundbreaking, safe, and simple strategy that can be applied in the clinical setting.
References
Gueler F, Gwinner W, Schwarz A et al, Long-term effects of acute ischemia and reperfusion injury. Kidney Int 2004;66:523–527.
Locke JE, Segev DL, Warren DS et al, Outcomes of kidneys from donors after cardiac death: implications for allocation and preservation. Am J Transplant 2007;7:1797–1807.
Summers DM, Johnson RJ, Allen J et al, Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010;376:1303–1311.
Wolfe RA, Merion RM, Roys EC et al, Trends in organ donation and transplantation in the United States, 1998-2007. Am J Transplant 2009;9 (4 Pt 2):869–878.
Zaouali MA, Ben Abdennebi H, Padrissa-Altes S et al, Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin Pharmacother 2010;11:537–555.
Bauer I, Pannen BH . Bench-to-bedside review: carbon monoxide—from mitochondrial poisoning to therapeutic use. Crit Care 2009;13:220.
Hatayama N, Naito M, Hirai S et al, Preservation by desiccation of isolated rat hearts for 48hours using carbon monoxide (PCO=4,000 hPa) and oxygen (PO(2)=3,000 hPa). Cell Transplant 2012;21:609–615.
Azuma H, Isaka Y, Li X et al, Superagonistic CD28 antibody induces donor-specific tolerance in rat renal allografts. Am J Transplant 2008;8:2004–2014.
Imamura R, Isaka Y, Sandoval RM et al, Intravital 2-photon microscopy assessment of renal protection efficacy of siRNA for p53 in experimental rat kidney transplantation models. Cell Transplant 2010;19:1659–1670.
Yoshida Y, Hatayama N, Seki K . Study on the preservation with CO (PCO=200-2,000 hPa), resuscitation, and heterotopic transplantation of an isolated rat heart. Cell Transplant 2009;18:535–540.
Hatayama N, Yoshida Y, Seki K . 72-Hour preservation, resuscitation, and transplantation of an isolated rat heart with high partial pressure carbon monoxide gas (PCO=400 hPa) and high partial pressure carbon dioxide (PCO2=100 hPa). Cell Transplant 2010;19:707–712.
Yamada K, Miwa T, Liu J et al, Critical protection from renal ischemia reperfusion injury by CD55 and CD59. J Immunol 2004;172:3869–3875.
Morita M, Fujino M, Jiang G et al, PD-1/B7-H1 interaction contribute to the spontaneous acceptance of mouse liver allograft. Am J Transplant 2010;10:40–46.
Kumar S, Bandyopadhyay U . Free heme toxicity and its detoxification systems in human. Toxicol Lett 2005;157:175–188.
Nakao A, Sugimoto R, Billiar TR et al, Therapeutic antioxidant medical gas. J Clin Biochem Nutr 2009;44:1–13.
Zhang X, Shan P, Alam J et al, Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem 2005;280:8714–8721.
Faleo G, Neto JS, Kohmoto J et al, Carbon monoxide ameliorates renal cold ischemia-reperfusion injury with an upregulation of vascular endothelial growth factor by activation of hypoxia-inducible factor. Transplantation 2008;85:1833–1840.
Nakao A, Faleo G, Shimizu H et al, Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int 2008;74:1009–1016.
Yoshida J, Ozaki KS, Nalesnik MA et al, Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am J Transplant 2010;10:763–772.
Sener A, Tran KC, Deng JP et al, Carbon monoxide releasing molecules inhibit cell death resulting from renal transplantation related stress. J Urol 2013;190:772–778.
Ruan Y, Wang L, Zhao Y et al, Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int 2014;86:525–537.
Martins PN, Reutzel-Selke A, Jurisch A et al, Induction of carbon monoxide in donor animals prior to organ procurement reduces graft immunogenicity and inhibits chronic allograft dysfunction. Transplantation 2006;82:938–944.
Otterbein LE, Bach FH, Alam J et al, Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 2000;6:422–428.
Datta SR, Brunet A, Greenberg ME . Cellular survival: a play in three Akts. Genes Dev 1999;13:2905–2927.
Noiri E, Nakao A, Uchida K et al, Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 2001;281:F948–F957.
Rodrigo R, Bosco C . Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol 2006;142:317–327.
Brugger J, Schick MA, Brock RW et al, Carbon monoxide has antioxidative properties in the liver involving p38 MAP kinase pathway in a murine model of systemic inflammation. Microcirculation 2010;17:504–513.
Hegazi RA, Rao KN, Mayle A et al, Carbon monoxide ameliorates chronic murine colitis through a heme oxygenase 1-dependent pathway. J Exp Med 2005;202:1703–1713.
Lee BS, Heo J, Kim YM et al, Carbon monoxide mediates heme oxygenase 1 induction via Nrf2 activation in hepatoma cells. Biochem Biophys Res Commun 2006;343:965–972.
t Hart NA, van der Plaats A, Faber A et al, Oxygenation during hypothermic rat liver preservation: an in vitro slice study to demonstrate beneficial or toxic oxygenation effects. Liver Transpl 2005;11:1403–1411.
Von Burg R . Carbon monoxide. J Appl Toxicol 1999;19:379–386.
Hatayama N, Inubushi M, Naito M et al, Functional evaluation of rat hearts transplanted after preservation in a high-pressure gaseous mixture of carbon monoxide and oxygen. Sci Rep 2016;6:32120.
Acknowledgements
The authors are grateful to Astellas Pharma for supplying the UW solution (Viaspan) and thank Mizuki Takeyama for her invaluable technical assistance. This study was supported by research grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grants-in-Aid 15K20087, 15K10043, 15K20303, 15F15756, 20390349, 21659310, 2109739, 15K10043, 15K20303, 15F15756).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The authors investigated a new strategy for organ preservation using ex vivo application of high-pressure carbon monoxide (CO) and oxygen in rat kidney transplantation model. High-pressure CO significantly improves graft function compared with simple cold storage using University of Wisconsin solution by protecting tubular epithelial cells from inflammation and apoptosis.
Rights and permissions
About this article
Cite this article
Abe, T., Yazawa, K., Fujino, M. et al. High-pressure carbon monoxide preserves rat kidney grafts from apoptosis and inflammation. Lab Invest 97, 468–477 (2017). https://doi.org/10.1038/labinvest.2016.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2016.157