Abstract
Renal prostaglandin (PG) E2 regulates salt and water transport, and affects disease processes via EP1–4 receptors, but its role in the proximal tubule (PT) is unknown. Our study investigates the effects of PGE2 on mouse PT fluid reabsorption, and its role in growth, sodium transporter expression, fibrosis, and oxidative stress in a mouse PT cell line (MCT). To determine which PGE2 EP receptors are expressed in MCT, qPCR for EP1–4 was performed on cells stimulated for 24 h with PGE2 or transforming growth factor beta (TGFβ), a known mediator of PT injury in kidney disease. EP1 and EP4 were detected in MCT, but EP2 and EP3 are not expressed. EP1 was increased by PGE2 and TGFβ, but EP4 was unchanged. To confirm the involvement of EP1 and EP4, sulprostone (SLP, EP1/3 agonist), ONO8711 (EP1 antagonist), and EP1 and EP4 siRNA were used. We first show that PGE2, SLP, and TGFβ reduced H3-thymidine and H3-leucine incorporation. The effects on cell-cycle regulators were examined by western blot. PGE2 increased p27 via EP1 and EP4, but TGFβ increased p21; PGE2-induced p27 was attenuated by TGFβ. PGE2 and SLP reduced cyclinE, while TGFβ increased cyclinD1, an effect attenuated by PGE2 administration. Na-K-ATPase α1 (NaK) was increased by PGE2 via EP1 and EP4. TGFβ had no effect on NaK. Additionally, PGE2 and TGFβ increased fibronectin levels, reaching 12-fold upon co-stimulation. EP1 siRNA abrogated PGE2-fibronectin. PGE2 also increased ROS generation, and ONO-8711 blocked PGE2-ROS. Finally, PGE2 significantly increased fluid reabsorption by 31 and 46% in isolated perfused mouse PT from C57BL/6 and FVB mice, respectively, and this was attenuated in FVB-EP1 null mice. Altogether PGE2 acting on EP1 and EP4 receptors may prove to be important mediators of PT injury, and salt and water transport.
Similar content being viewed by others
Main
Prostaglandin (PG) E2 is a major product of cyclooxygenase activity in the kidney. It has a substantial role in maintaining hemodynamics, salt and water homeostasis, and affects growth, inflammation, oxidative stress, and fibrotic responses (reviewed in refs. 1, 2, 3). Four EP receptors (EP1–4) mediate the signalling responses to PGE2, by altering intracellular cAMP and/or Ca2+ levels. Renal cells often simultaneously express multiple EP receptors, and their relative levels determine the cell’s response. Although the contribution of the proximal tubule (PT) to overall renal prostaglandin synthesis is minimal, the role of PGE2 in PT transport function has been considered. For instance, PGE2 stimulates cAMP and activates protein kinase A, and in turn regulates basolateral organic anion uptake.4, 5 In contrast, long-term exposure to PGE2 inhibits its excretion by decreasing the levels of basolateral organic anion transporters that are responsible for PGE2 uptake in rat renal PT cells.6 The underlying regulatory pathways are not completely understood, but short-term vs long-term exposure to PGE2 has opposite effects on the overall cell response. PGE2 also reduces phosphate transport in mouse proximal convoluted tubules.7 Though very little is known about PT salt and water transport in response to PGE2, a recent study by Herman et al8 describes a mechanism involving both protein kinase C and cAMP, by which PGE2 regulates Na+-K+-ATPase in primary cultures of rabbit renal PT cells. PGE2 can also mediate the responses of other hormonal systems on PT sodium transport function. For instance, Lopes et al9 showed that Angiotensin-(1–7)-induced Na+-ATPase activity is inhibited by bradykinin via PGE2. Though it is clear that PGE2 could influence the transport properties of the PT, to date the specific contribution of each PGE2/EP receptor pathway to these responses has not been thoroughly investigated.
In addition to its role in regulating tubular transport, PGE2 contributes to injurious processes in a variety of renal cells, and thereby influences the initiation and progression of kidney injury. The PT is the primary tubular segment affected in a number of kidney diseases. A recent emphasis is placed on the role of the tubulointerstitium in kidney disease, and its contribution to both initial events perturbing kidney function, but also in sustaining renal injury.10, 11, 12 A tubular hypothesis whereby PT growth promotes PT hyper-reabsorption, which in turn contributes to hyperfiltration and hypertension, leading to further renal injury, is now given significant recognition in the literature (reviewed in Vallon and Thomson12). Transforming growth factor beta (TGFβ) is a key regulator of PT changes, promoting and maintaining alterations in growth and fibrotic responses.13, 14 Since PGE2 regulates renal growth and tubular transport, it is essential to examine the contribution of specific PGE2/EP receptor pathways to PT injury and clarify the effects on salt and water transport in this segment of the nephron. A study by Mohamed et al15 showed that COX-2 derived PGE2 is elevated in the PT and mediates inflammatory and apoptotic responses that contribute to injury in streptozotocin diabetic mice, and this is alleviated with netrin-1 overexpression. The exact mechanisms were not clarified. But overall, in the kidney PGE2 acting on EP4 receptors prevents injury,16, 17, 18 whereas EP1 contributes to injurious events.19, 20 Our study investigates the effects of PGE2 on PT water transport, growth, sodium transporters, fibrosis, and ROS generation, and the interaction with TGFβ-mediated responses in a mouse PT cell line (MCT). Having confirmed that only EP1 and EP4 receptors are expressed in MCT cells, various approaches to distinguish EP1 vs EP4 mediated responses were employed, including: comparisons between PGE2 and the EP1/3 agonist sulprostone (SLP), EP1 antagonist ONO8711, and EP1 and EP4 siRNA. A better understanding of the specific contribution of EP1 and EP4 receptors could prove to be important for kidney disease intervention strategies.
MATERIALS AND METHODS
MCT Cell Culture
The mouse renal tubular epithelial cell line (MCT) was a generous gift from Dr Eric Neilson (Northwestern University, Chicago, IL). These cells were characterized and shown to possess many of the characteristics of the in vivo PT.21 MCT cells were grown in DMEM: F-12 (1:1) media supplemented with 10% FBS, 1% Penicillin-Streptomycin, and 1% L-glutamine and were maintained at 5% CO2 and 37 °C during culture and treatment. Sub-confluent cells were maintained in serum-free media for 24 h and stimulated with 1 μM PGE2 and 2 ng/ml of TGFβ for 24 h. If a response to 1 μM PGE2 was observed, MCT cells were then stimulated for 24 h with 1 nM PGE2 as well as 1 μM and 1 nM SLP (Cayman, Ann Arbor, MI, USA). Sulprostone is an EP1/3 receptor agonist, but since EP3 receptors are not expressed in MCT cells (as indicated below), SLP was used to determine the involvement of EP1 receptors in the various responses. EP1 receptors were antagonized with 100 nM ONO-8711 (Cayman) or blocked by EP1 siRNA transfections, EP4 receptors were blocked with EP4 siRNA transfections (see below).
EP siRNA Transfections
MCT cells were cultured for 24 h and then transient transfections with EP1 or EP4 siRNA (Qiagen, Germany) were carried out as previously described.22 Briefly, EP siRNA was prepared according to the manufacturer’s instructions to a final concentration of 5 nM in 100 μl DMEM-F12 and HiPerfect Transfection reagent (Qiagen), and cells were maintained for a total of 48 h. A universal scrambled siRNA (Qiagen) was used as a negative control for these experiments. PGE2 stimulations (1 μM) were carried out during the final 24 h transfection. Protein lysates were isolated, as well as total RNA to confirm the inhibition of EP1 and EP4 following siRNA transfections by quantitative PCR, as described below.
RNA Isolation and Quantitative PCR
Total RNA was isolated from MCT with TRIzol reagent (Life Technologies, Grand Island, NY, USA) and treated with DNAse I (Life Technologies). Quantitative PCR was performed to measure EP1–4 receptors in samples using specific Taqman probes and primers from Applied Biosystems using the TaqMan One-step RT-PCR master mix reagents and the ABI Prism 7000 sequence detection system as previously described.23 A 6-point standard curve was performed for each experiment as a control using mouse cortex RNA from 4.7 to 100 ng. Expression was normalized to GAPDH, detected with the TaqMan Rodent GAPDH control reagent kit (Life Technologies).
3H-Thymidine and 3H-Leucine Incorporation
To study the effects of PGE2 and TGFβ on cell DNA and protein synthesis, we measured the incorporation of 3H-thymidine and 3H-leucine, respectively. MCT cells were cultured in 24-well plates, grown to 70% confluence and then cultured in serum-free media for 24 h stimulated with 1 μM or 1 nM PGE2 or SLP, or 2 ng/ml of TGFβ (R&D Systems, Minneapolis, MN, USA). 3H-thymidine or 3H-leucine (0.5 μCi/ml) was added to each well for the duration of the stimulation. The plates were then washed four times in ice-cold PBS. Next, the cells were permeabilized in 500 μl of 1 N NaOH at 37 °C for 30 min, and the amount of 3H-thymidine or 3H-leucine, in counts per minute, was measured using a scintillation counter. Samples were done in triplicate and incorporation was expressed as fold of control.
Western Blotting
Confluent MCT cells were lysed in RIPA buffer containing: 0.5 mM PMSF, 1% protease inhibitor cocktail, 1 mM sodium pyrophosphate, 10 mM sodium fluoride and 100 μM sodium orthovanadate, and sonicated for 5 s. Protein was quantified with Bradford reagent (Bio-Rad, Hercules, CA, USA). Samples were denatured at 70 °C for 15 min, electrophoresed and transferred onto a nitrocellulose membrane. Membranes were blocked in 10% milk/TBS-T for 90 min and incubated overnight with primary antibody followed by secondary antibody for 90 min. The following antibodies were used: anti-p27 (Calbiochem, San Diego, CA, USA), anti-p21 (Santa Cruz, Dallas, TX, USA), anti-cyclinD1 and cyclinE (Santa Cruz), anti-Na-K-ATPase-α1 (Millipore Canada, Etobicoke, ON, Canada), and anti-fibronectin (Sigma-Aldrich, St Louis, MO, USA). Super Signal West Pico Chemiluminescent reagents (Thermo Scientific, Rockford, IL, USA) were applied and β-actin was detected as a loading control for densitometry.
Lucigenin Assay
The lucigenin assay was used to measure ROS production in MCT cells in response to PGE2. Cultured cells were stimulated for 24 h and scraped in lysis buffer containing KH2PO4, EGTA, aprotinin, pepstatin, leupeptin, and PMSF. In all, 50 μl of each sample was assayed using the lucigenin-derived chemiluminescence assay, in 175 μl assay buffer (KH2PO4, EGTA, and Sucrose) containing 1 mM lucigenin. Following basal measurements for each sample in a 96-well plate, NADPH (0.1 mM) was added to each well and luminescence was measured every 1.8 s for 30 cycles in a microplate luminometer (Orion II, Berthold Detection System), using the Simplicity 4.2 Program as previously described.24 Activity was expressed as relative chemiluminescence units per μg protein. Apocynin (50 μM) was used to inhibit NADPH oxidase as previously done in our laboratory.24
Microdissections of PTs
Male C57BL/6 and FVB mice 4–6 months of age were killed by decapitation. The kidneys were quickly removed, and 1–2 mm coronal slices were placed in chilled dissection dishes for freehand dissection of PTs, distinguished from other segments based on various properties: diameter difference, cell heterogeneity, and transluscency.21 The microdissected PTs were then transferred to a chamber for in vitro perfusions and measurement of net fluid reabsorption (Jv). To confirm the involvement of EP1 receptors in fluid reabsorption, PTs were also isolated from mice lacking the EP1 receptor (FVB-EP1 null mice), which our group generated and thoroughly characterized.20
Measurement of Net Fluid Reabsorption (Jv)
In vitro microperfusion of isolated mouse PTs was performed as previously described.25, 26 Microdissected tubules (described above) were transferred to a thermostatically controlled chamber of 1 cm3 volume and cannulated using concentric micropipettes. Bath solution was continuously exchanged at 0.5 ml/min by infusion pump (Harvard Apparatus, Holliston, MA, USA) and was maintained at 37 °C. The dissecting solution consisted of (in mM): NaCl, 137; MgCl2, 1; MgSO4, 0.8; KCl, 5; CaCl2, 0.25; Tris-HCl, 10; Na2HPO4, 0.33; glutamine, 2; KH2PO4, 0.44; and L-lactate, 2. The perfusate composition was (in mM): NaCl, 125; NaHCO3, 22; KCl, 5; CaCl2, 1; MgSO4, 1.2; glucose, 10.5; glutamine, 2; L-lactate, 2; and phosphoric acid, 1.2. The composition of bath medium was (in mM) NaCl, 101; NaHCO3, 22; KCl, 5; CaCl2, 1; MgSO4, 1.2; glucose, 10.5; glutamine, 2; L-lactate, 2; phosphoric acid, 1.2, and Hepes, 32.5 (pH 7.4, osmolality 300 mOsmol). Bovine serum albumin (5%, Sigma, St Louis, MO, USA) was also added before dissections. The perfusate, which contained H3-inulin (75 μCi/ml) as a volume marker, was collected into a constriction pipette of known volume (between 90 and 130 nl) and counted for H3-inulin (New England Nuclear, Boston, MA, USA). The perfusion rate was maintained between 12 and 20 nl/min by adjusting the hydrostatic pressure. At this perfusion rate, osmotic equilibration between bath and lumen does not occur. In control studies, 30 min of equilibration was allowed and then, three collections were made for calculation of basal Jv in nl/mm/min. Tubules with a negative basal Jv were discarded. Then, 0.1 μM or 1 nM PGE2 (Cayman) was added to the bath and 5 timed collections were made to determine net volume reabsorption (Jv). In the experimental period, the three highest collections of five were used to calculate mean Jv as the difference between the perfusion rate Vo and the collection rate VL, both in nl/min, normalized to tubule length (L, in mm): Jv=(Vo−Vo)/L, where Vo=VL (CL/Co), where CL and Co are perfusate and collected fluid concentrations in cpm/nl, respectively.
Statistics
GraphPad Prism v4.03 (La Jolla, CA, USA) was used to plot and analyze the data. Values are expressed as means±standard error of the mean (s.e.m.). A one-way ANOVA was performed with Tukey post-test. We also used an unpaired t-test to assess statistical significance between selected experimental groups. Additionally, a one-sample unpaired t-test was performed against a hypothetical value of 1.0 for all groups as the values represented fold controls, where the control group all had values of 1.0, P<0.05.
RESULTS
EP1 Receptors are Increased in MCT by PGE2 and TGFβ
As shown in Figure 1a–d, both EP1 and EP4 receptors are expressed in MCT cells. EP2 and EP3 subtypes were not detected in MCT cells, but were detectable previously with the same probes and primers in other renal cell lines and mouse cortex and medulla samples.23 In our experiment a standard curve was produced using mouse cortex as a control, further confirming the lack of expression of these two receptors in the MCT samples. The expression of EP1 and EP4 receptors in response to 24 h PGE2 and TGFβ treatments were further characterized. Both PGE2 and TGFβ increased EP1/GAPDH by 2.5- and 3.8-fold, respectively (Figure 1a), but EP4 receptors remained unchanged (Figure 1c). Co-stimulation with both PGE2 and TGFβ resulted in a 7-fold increase in EP1 receptor expression, which was significantly greater than TGFβ alone, but not greater than the response to PGE2 alone. Transfection of MCT cells with EP1 (Figure 1b) and EP4 (Figure 1d) siRNA resulted in a 50 and 75% reduction of EP1 and EP4 mRNA levels, respectively. However, scrambled siRNA had no effect on EP1 or EP4. EP1 siRNA also reduced the PGE2-mediated increase in EP1 receptors (P=0.06) back to control levels (Figure 1b).
PGE2 and TGFβ increase EP1 but not EP4 expression in MCT cells. MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and EP1 (a) and EP4 (c) receptors were detected by quantitative PCR. MCT cells were transfected for 48 h with EP1 (b) and EP4 (d) siRNA. EP/GAPDH presented as mean±s.e.m., fold of control (C=1), n=6 and 8, respectively. *P<0.05 vs C; &P<0.05 vs T or P. EP1si or EP4si, EP1 or EP4 siRNA (5 nM); P, PGE2 (1 μM); P+EP1si, PGE2 and EP1 siRNA; P+EP4si, PGE2 and EP4 siRNA; Ssi, scrambled siRNA; P+Ssi, PGE2 and scrambled siRNA; T, transforming growth factor β (TGFβ, 2 ng/ml); TP, TGFβ+PGE2.
PGE2 Inhibits Cell-Cycle Progression in MCT Cells
To study the effect of PGE2 on MCT growth responses, we first examined 3H-thymidine and 3H-leucine incorporation. As shown in Figure 2, 3H-thymidine incorporation (Figure 2a) was reduced by 47 and 68% in response to PGE2 and TGFβ, respectively, and reduced by 76% upon co-stimulation. Though the co-treatment was significantly less than the inhibition by PGE2 alone, the inhibition upon co-treatment was not significant compared with TGFβ alone. Since EP3 receptors were not detected in MCT cells (discussed above), SLP was used as an EP1 selective agonist to determine the extent of EP1 involvement. As shown in Figure 2c, SLP, like PGE2, also reduced thymidine incorporation by about 50%, and this effect was not influenced by the concentration of PGE2 or SLP used (1 μM vs 1 nM treatments). Similarly, H3-leucine incorporation (Figure 2b) was reduced by 40 and 65% in response to PGE2 and TGFβ, and by 73% upon co-stimulation. The effect of co-treatment with PGE2 and TGFβ was statistically significant compared with PGE2 alone, but not different from TGFβ alone. Sulprostone and 1 nM PGE2 reduced leucine incorporation by 25% (Figure 2d). To further explore the mechanism of growth inhibition, we examined the effects of PGE2 on cell-cycle regulators, compared with TGFβ. Both p27 and p21 inhibit the cell cycle as cyclin-dependent kinase inhibitors,27 and are key regulators of growth responses in renal cells. A representative blot for each and the corresponding β-actin are shown (Figure 3). As seen in Figure 3a, 1 μM PGE2 increased p27 levels by 1.75-fold and SLP by 1.5-fold (Figure 3c). Transfection of MCT cells with EP4 siRNA (Figure 3d) and EP1 siRNA (Figure 3e) attenuated the p27 response to PGE2, though only significant with the EP4 siRNA (Figure 3d). The scrambled siRNA however had no effect on p27 levels, though the co-stimulation with PGE2 and scrambled siRNA was only numerically increased by 1.5-fold (Figure 3d). Interestingly, EP4 siRNA alone reduced p27 levels by 35% (Figure 3d), but EP1 siRNA alone had no effect (Figure 3e). In contrast, TGFβ reduced p27 by 56% (Figure 3a), and significantly attenuated the response to PGE2 upon co-stimulation. PGE2 did not affect p21 levels (Figure 3b), but TGFβ increased p21 levels by 4.4-fold (Figure 3b). The PT growth responses are also regulated by cyclinD1/CDK4 and cyclinE/CDK2 complexes.10 While PGE2 did not alter cyclin D1 on its own, TGFβ increased cyclinD1 by 4.6-fold and PGE2 attenuated this response to 1.6-fold (Figure 4a). PGE2 did however decrease cyclinE by 25% (Figure 4b), whereas TGFβ had no effect on cyclinE levels. Similarly, SLP and 1 nM PGE2 reduced cyclinE levels by 50–75% (Figure 4c). A representative blot for each of cyclinD1 and cyclinE, and their corresponding β-actin, are also shown in Figure 4.
PGE2 inhibits DNA and protein synthesis in MCT cells. MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and DNA and protein synthesis were assessed by 3H-thymidine (a) and 3H-leucine (b) incorporation. Comparisons were made between PGE2 and SLP, at 1 μM or 1 nM for thymidine (c) and leucine (d). Data presented as mean ± s.e.m., fold control (C=1). *P<0.05 vs C, #P<0.05 vs P, n=5. C, control; P, PGE2 (1 μM); PGE2 1 μM (μP) and 1 nM (nP); SLP 1 μM (μS) and 1 nM (nS); T, transforming growth factor β (TGFβ, 2 ng/ml); TP, TGFβ+PGE2.
PGE2 increases p27 levels in MCT cells via EP1 and EP4 receptors. MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and p27 (a) and p21 (b) levels were assessed by western blotting. Representative blots of p27 or p21 (upper) and corresponding β-actin (lower) are shown for each experiment. In (c), MCT cells were treated with PGE2 and the SLP (1 μM or 1 nM), and the densitometric analysis of p27/β-actin is shown. In (d), MCT cells were transfected for 48 h with EP4 siRNA (EP4si) or a universal scrambled siRNA (Ssi), and PGE2 was added during the final 24 h. In (e), MCT cells were transfected with EP1 siRNA (EP1si). The densitometric analysis is shown with data presented as mean ± s.e.m., fold control (C=1). *P<0.05 vs C, &P<0.05 vs P, n=4–8. C, control; EP1si or EP4si, EP1 or EP4 siRNA (5 nM); P, PGE2 (1 μM); PGE2 1 μM (μP) and 1 nM (nP); SLP 1 μM (μS) and 1 nM (nS); P+EP1si, PGE2 and EP1 siRNA; P+EP4si, PGE2 and EP4 siRNA; P+Ssi, PGE2 and scrambled siRNA; Ssi, scrambled siRNA; T, transforming growth factor β (TGFβ, 2 ng/ml); TP, PGE2 and TGFβ.
PGE2 and SLP reduce cyclin E expression in MCT cells. MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and cyclin D1 (a) and cyclin E (b) levels were assessed by western blotting. In (c), MCT cells were treated with PGE2 and SLP (1 μM or 1 nM), and cyclin E levels were assessed. A representative blot of cyclin (upper) and β-actin (lower) is shown for each. The densitometric analysis is presented with data expressed as mean ± s.e.m., fold control (C=1). *P<0.05 vs C, &P<0.05 vs T, n=6. C, control; P, PGE2 (1 μM); PGE2 1 μM (μP) and 1 nM (nP); SLP 1 μM (μS) and 1 nM (nS); T, transforming growth factor β (TGFβ, 2 ng/ml); TP, TGFβ+PGE2.
PGE2 Increases the Expression of Sodium Transporters in MCT Cells and Stimulates PT Fluid Reabsorption
PGE2 has an important role in regulating salt and water transport along the nephron, but its effect on PT sodium and water transport is unknown. In MCT cells, PGE2 increased Na+-K+-ATPase α1 (NaK) subunit by 2.4-fold (Figure 5a), whereas TGFβ alone had no effect. However, TGFβ significantly attenuated the stimulatory effect of PGE2 to 0.5-fold. A representative blot showing NaK and the corresponding β-actin is shown for each experiment (Figure 5). Sulprostone (1 μM) only numerically increased NaK levels by 1.35-fold, P>0.05 (Figure 5b). Transfection of MCT cells with EP4 siRNA abrogated the PGE2-mediated NaK response from 1.95-fold to 1.3-fold, though not statistically significant (Figure 5c). Scrambled siRNA had no effect on NaK levels, and the PGE2 effect in the presence of the scrambled siRNA was unchanged at 1.87-fold. As shown in Figure 5d, EP1 siRNA also attenuated the PGE2-induced NaK response to 1.2-fold. The majority of PT sodium transport is mediated by the sodium-hydrogen exchanger, NHE1 on the basolateral side and NHE3 on the apical membrane.28 Unlike the increase in basolateral NaK shown here, NHE1 and NHE3 levels were unchanged following 24 h PGE2 (unpublished data).
PGE2 increases sodium potassium ATPase expression in MCT cells via EP1 and EP4 receptors. In (a), MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and sodium potassium ATPase α1 (NaK) levels were assessed by western blotting. A representative blot is shown for NaK (upper) and corresponding β-actin (lower) for each experiment. The densitometric analysis of NaK/β-actin is shown with data presented as mean ± s.e.m., fold control (C=1). In (b), MCT cells were treated with PGE2 and SLP (1 μM or 1 nM). In (c), MCT cells were transfected for 48 h with EP4 siRNA (EP4si) or scrambled siRNA (Ssi), and PGE2 was added during the final 24 h. In (d), MCT cells were transfected with EP1 siRNA (EP1si). C, control; EP1si or EP4si, EP1 or EP4 siRNA (5 nM); P+ EP1si, PGE2 and EP1 siRNA; P+EP4si, PGE2 and EP4 siRNA; P, PGE2 (1 μM); PGE2 1 μM (μP) and 1 nM (nP); SLP 1 μM (μS) and 1 nM (nS); P+Ssi PGE2 and scrambled siRNA; Ssi, scrambled siRNA (5 nM); T, transforming growth factor β (TGFβ, 2 ng/ml); TP, PGE2 and TGFβ. *P<0.05 vs C, &P<0.05 vs P, n=4–8.
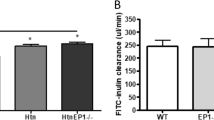
As shown in Figure 6, in isolated perfused mouse proximal convoluted tubules from C57BL/6 mice, PGE2 increased net fluid reabsorption (Jv) in response to 1 nM and 0.1 μM PGE2 (post-treatment), by 119% (n=5, P=0.07) and 131% (n=5, P<0.05) of control (C=100, pre-treatment), respectively (Figure 6a). The time course control was unchanged. In FVB mice, 0.1 μM PGE2 stimulated Jv by 146% of control (C=100, P<0.05), and this effect was completely abolished in mice lacking EP1 receptors to 96% of control (Figure 6b).
PGE2 increases fluid reabsorption in mouse proximal tubules via EP1 receptors. In (a), mouse isolated perfused tubules from C57BL/6 mice were treated with PGE2 (10–9 and 10−7 M), and net fluid reabsorption (Jv: nl/mm/min) was determined. A time control is also shown (Control). Data are shown as mean ± s.e.m. of the three highest collections pre- (untreated, white bars) and post-PGE2 treatment (black bars), and expressed as % of pre-treatment control (pre, white bars) which is presented at 100%. N=5. *P<0.05 vs respective pre-treatment. In (b), tubules were obtained from FVB heterozygous (EP1 +/−) or mice lacking EP1 receptors (EP1−/−), and Jv is presented as mean ± s.e.m. for untreated (control, white bars) and PGE2-treated tubules (black bars). The percentage of control (C=100) for n=4 and 5 tubules is shown, respectively. Results of paired t-test analysis are indicated. *P<0.05 vs control.
PGE2 Increases Fibronectin in MCT Cells Via EP1 Receptors
To further characterize the PGE2 response in MCT cells, the effect of PGE2 upon fibronectin levels was assessed as previously described.29 As shown in Figure 7a, fibronectin levels were significantly increased in response to PGE2 and TGFβ stimulation for 24 h, 1.9- and 6.5-fold, respectively, and then upon co-stimulation a greater response was observed, reaching 12-fold (Figure 8a). This was significantly greater than the response to PGE2 alone, but not that of TGFβ alone. EP1 siRNA reduced fibronectin, and significantly attenuated the response to PGE2 (Figure 7b). As shown scrambled siRNA had no effect on fibronectin levels or the stimulatory response to PGE2. On the other hand, EP4 siRNA had the opposite effect of EP1 siRNA, slightly increasing fibronectin by 1.55-fold (not statistically significant), and numerically enhanced the PGE2 response from 2.1-fold to 2.96-fold (Figure 7c). Though TGFβ increased NFκB/GAPDH by almost 2-fold, PGE2 had no effect on nuclear factor κB mRNA (Figure 7d).
PGE2 increases fibronectin levels in MCT cells via EP1 receptors. MCT cells were stimulated for 24 h with TGFβ and PGE2 in the presence or absence of TGFβ, and fibronectin levels (fibro) were assessed by western blotting (a). A representative blot is shown for fibronectin (upper) and corresponding β-actin (lower), and the densitometric analysis of fibro/β-actin is shown for each experiment. In (b), MCT cells were transfected for 48 h with EP1 siRNA (EP1si) or scrambled siRNA (Ssi), and PGE2 was added during the final 24 h. In (c), MCT cells were transfected with EP4 siRNA (EP4si). In (d), following 24 h stimulations, NFκB expression was assessed by quantitative PCR and normalized to GAPDH. Data are presented as mean ± s.e.m., fold control (C=1). *P<0.05 vs C, &P<0.05 vs P. N=4–7. C, control; EP1si or EP4si, EP1 or EP4 siRNA (5 nM); NFκB, nuclear factor kappa B; P, PGE2 (1 μM); P+EP1si, PGE2 and EP1 siRNA; P+EP4si, PGE2 and EP4 siRNA; P+Ssi PGE2 and scrambled siRNA; Ssi, scrambled siRNA (5 nM); T, transforming growth factor β (TGFβ, 2 ng/ml); TP, TGFβ+PGE2.
PGE2 increases ROS generation in MCT cells via EP1 receptors. Lucigenin assay following 24 h stimulations of MCT cells. Data are presented as relative chemiluminescence units per μg of protein. The mean ± s.e.m. are shown as fold control (C=1). *P<0.05, n=5. apo, apocynin (NADPH oxidase inhibitor; C, control (DMEM); I, indomethacin (cyclooxygenase inhibitor; 10 μM); O, ONO-8711 (EP1 antagonist; 100 nM); PI, PGE2 (1 μM) + indomethacin; PIO, PGE2+indomethacin+ONO-8711; 50 μM); PI+apo, PGE2+indomethacin+apocynin; PIO+apo, PGE2+indomethacin+ONO-8711+apocynin.
PGE2 Increases ROS Generation in MCT Cells Via EP1 Receptors
Since PGE2 acting on EP1 receptors increased fibronectin, and oxidative stress responses have a key role in the fibrotic process in PTs,10, 30, we studied whether EP1 receptors are involved in ROS generation using the lucigenin-enhanced chemiluminescence assay. As shown in Figure 8, stimulation for 24 h with PGE2 increased ROS production in MCT cells by 1.8-fold; and this response was attenuated by the EP1 antagonist ONO-8711 to 1.2-fold. Apocynin a known inhibitor of NADPH oxidase completely abrogated the lucigenin response, suggesting that NADPH oxidase may be the source of ROS production by PGE2.
DISCUSSION
In the kidney, PGE2 is recognized for its vascular effects and glomerular hemodynamic responses, its role in distal tubule and collecting duct transport, and renin secretion at the level of the macula densa (reviewed in Nasrallah et al31). We examined whether EP receptors are present in the MCT and characterized the responses to PGE2. The major findings are that MCT cells only express EP1 and EP4 receptors, and EP1 is increased by both PGE2 and TGFβ. An inhibition of the cell cycle (DNA and protein synthesis) was observed in response to PGE2 and TGFβ in MCT cells, but this was achieved through distinct pathways: p27 vs p21, and cyclinE vs cyclinD1, respectively. Figure 9 summarizes the MCT growth response to PGE2, upon activation of EP1 or EP4 receptors, compared with TGFβ. PGE2 and TGFβ also have opposite effects on sodium transporters: PGE2 increases the levels of NaK via EP1 and EP4, and the PGE2 response is attenuated by TGFβ. In the isolated perfused PT, PGE2 increased fluid reabsorption via EP1 receptors. PGE2 also increases MCT cell fibronectin and ROS generation via EP1.
PGE2 and TGFβ inhibit growth responses in MCT cells. As depicted in this summary, PGE2 and TGFβ affect different cell-cycle regulators in MCT cells to inhibit protein and DNA synthesis. In proximal tubule cells, growth responses are mainly regulated by cyclin-dependent kinase inhibitors p27 and p21, or cyclinD1/CDK4 and cyclinE/CDK2 complexes. Our study shows that in MCT cells PGE2 binds cell surface EP1 or EP4 receptors to increase p27 and reduce cyclinE, whereas TGFβ increases p21 and cyclinD1. PGE2 attenuated the TGFβ-cyclinD1 response, and TGFβ attenuated the PGE2-p27 response. TGFβ also enhanced the growth inhibitory response of PGE2. But the overall effect is cell-cycle arrest.
Though EP receptor protein levels along the nephron have proven difficult to detect, relying mainly on pharmacological responses to selective agonists and antagonists, a study reported the expression of all four EP receptor subtypes in rabbit PTs, which were isolated with iron oxide to separate glomeruli.8 In our study, only EP1 and EP4 were detected in MCT cells at the mRNA level. Similarly, we previously isolated rat PTs by Percoll gradient and detected EP(1, 3, and 4) in the tubule suspensions, but when the suspensions were cultured under selective conditions for PT growth, the EP3 receptor was no longer detectable.32 Distal tubule contamination of the suspensions, that abundantly express EP3, probably accounts for the detection of EP3 receptors in these rat preparations, and could explain its detection in rabbit PTs,8 alternatively species differences that might account for the discrepancy.
In kidney disease, the PT undergoes changes in growth, and is subjected to oxidative stress that alters cell fate and promotes fibrosis. It is increasingly recognized that the tubular-interstitial alterations are associated with progression of diabetic kidney disease. In a recent review by Tang and Lai30 the pathogenic role of the PT epithelial cell and its activation by a number of substances is elaborately described, explaining the resulting cell-cycle arrest, inflammatory, and fibrotic responses, oxidative stress as well as altered transport function.10 In the PT, CDK2/cyclinE and CDK4/cyclinD1 regulate cell-cycle progression, and this is inhibited by cyclin-dependent kinase inhibitors p27 and p21.10 The contribution of PGE2 to regulation of cell cycle in healthy and injured renal cells has been described16, 33, 34, 35, 36 but not in the PT. Our work showed that PGE2 and SLP inhibit the cell cycle in MCT cells similar to TGFβ, reducing both protein and DNA synthesis. As depicted in Figure 9, the effects on regulators of cell-cycle progression in MCT cells were opposite for PGE2 and TGFβ. While PGE2 increased p27 via EP1 and EP4 receptors, TGFβ increased p21. Terada et al37 previously reported this same growth inhibitory response to 12 h TGFβ treatment in renal tubular LLC-PK1 cells, but mediated by decreased cyclin D1 levels rather than increased p21.
TGFβ is most often a proliferation inhibitor, especially in epithelial cells.38, 39, 40, 41, 42 Though our studies corroborate this effect, we also noted an increase in cyclinD1 levels in MCT cells stimulated with TGFβ. Since p21 binds CDK2 and inhibits the cell cycle, it is not unforeseen that cyclinD1 levels are transiently increased as part of a mechanism of cell adaptation to the quiescent state, but detailed time analysis is needed to confirm this possibility. Additionally, both PGE2 and SLP reduced cyclin E, and PGE2 attenuated TGFβ-mediated cyclinD1. Since transfection of MCT cells with EP1 and EP4 siRNA abrogated the increase in p27, the inhibition of the cell cycle in MCT cells is likely mediated by PGE2 acting on both EP1 and EP4 receptors to increase p27. However, the underlying mechanisms appear to be species and cell type specific (tubular epithelial cells vs vascular smooth muscle cell-like mesangial cells), and highly dependent on the culturing conditions. They may also be related to the concentration of PGE2 used, for instance, lower concentrations of PGE2 also increased p27 in rat mesangial cells causing cell-cycle arrest via the EP1 receptor, but increased protein synthesis.43 We did not observe a p27 response to 1 nM PGE2 in the MCT cells, though the inhibition of DNA synthesis was not concentration dependent and was noted with higher and lower concentrations of both PGE2 and SLP. Another interesting observation is that we only observed a senescent-like (reduced DNA and protein synthesis) response to PGE2 like TGFβ, without any cell hypertrophy. The typical hypertrophic response often described in kidney disease characterized by reduced DNA but increased protein synthesis is only observed under certain conditions in renal cells, and it appears to be delayed, requiring 48–96 h.44, 45 In a study looking at angiotensin II responses in PT cells, 48 h of angiotensin II treatment reduced thymidine but increased leucine incorporation. TGFβ also induces this hypertrophic response following longer exposure in PT cells.44, 45 Therefore, it is possible that this senescent-like state described in our 24 h study is transient, and PGE2 would have the same hypertrophic effect with longer stimulations. Further studies are needed to explore this possibility. Regardless of the exact mechanisms leading to cell-cycle arrest, the resultant senescence may have important implications in the context of renal disease progression, leading to inflammatory and fibrotic responses, and perturbed PT transport function (reviewed in Vallon and Thomson12). These findings lead to interesting avenues for future exploration.
It is proposed that PT growth promotes PT sodium hyper-reabsorption in chronic kidney disease, which in turn contributes to hyperfiltration and renal injury.46 Therefore, the contribution of PGE2/EP receptors to changes in salt and water transporters in the PT is of great interest. Our work indicates that PGE2 increases sodium transporter expression in PT cells via EP1- and EP4-dependent mechanisms. The increase in NaK is consistent with other reports showing that PGE2 stimulates NaK via PKA (conceivably mediated by EP4); however, PKC (probably EP1)-dependent mechanisms were also reported in mouse and rabbit PTs.8, 15 Similarly, the stimulation of PT sodium hydrogen exchanger (NHE3) expression by PGE2 was indirectly confirmed in a previous study showing that inhibition of COX-2 derived PGE2 in a model of rat experimental colitis, using COX-2 antisense oligonucleotides, attenuated the rise in PT NHE3 associated with the inflammatory state.47 We did not observe a change in NHE1 or NHE3 in response to PGE2 in our cells (unpublished data).
In addition to increasing NaK in MCT cells, PGE2 stimulated fluid reabsorption in isolated perfused mouse tubules. The mean response over baseline was 31% in C57BL/6 mice, and 46% in FVB mice; of importance, this is the first demonstration in mouse PT of a functional role for PGE2 in this segment. We also confirmed the role of EP1 receptors in the PGE2 mediated fluid reabsorption, since the stimulatory effect was absent in EP1 null tubules. However, we cannot rule out the involvement of EP4 receptors at this time since global knockouts of EP4 are not available due to lethality.48 Interestingly, we had previously reported a minimal response to PGE2 on baseline water transport in the isolated perfused rabbit cortical collecting duct. Only in the presence of vasopressin did PGE2 greatly affect the transport properties of the collecting duct.26 In a rat study of straight PT, Garvin49 demonstrated a 20% response in fluid transport with angiotensin II, but to the best of our knowledge there are no other studies looking at hormonally stimulated water transport in the mouse PT. Interestingly, the minimal responses in the PT may be due to shifting of transport between paracellular and transcellular pathways, so that by altering aquaporin-1 mediated transcellular transport in response to angiotensin II or PGE2, paracellular transport is reduced, and overall balance is maintained. A nice portrayal of these two fluid transport systems in the proximal has been elaborately presented by Schnermann et al,50 using claudin-2/aquaporin-1 double knockout mice, showing the shift from paracellular (claudin-2 dependent) to transcellular (AQP-1 dependent) transport in single (AQP-1 or claudin-2) knockouts to compensate for the lacking transport pathway. Another possibility is that in the healthy PT fluid transport is not very dependent on hormonal stimulation, and in the challenged (kidney disease) PT there is greater hormone dependence. Studies are ongoing in our laboratory to validate this possibility.
A common feature of progressive kidney disease is fibrosis induction, characterized by cytoskeletal reorganization, matrix changes, and scarring. TGFβ is the main growth factor reported to mediate the initiation and maintenance of the fibrotic process.14 In our study, we show that PGE2 increases fibronectin levels in mouse proximal tubule MCT cells, and EP1 but not EP4 receptors are involved in this response. Numerous studies implicate oxidative stress in the PT changes in response to high glucose, and reduction of ROS reduces fibrosis in this segment of the nephron.51 Our group29 recently demonstrated a role for NADPH oxidase NOX-4 in fibrotic events resulting from exposure of MCT cells to high glucose. PGE2 also increased ROS generation in MCT cells, and like fibronectin EP1 receptors mediate this effect. This finding is consistent with our recent work in diabetic EP1 receptor null mice, where cortical fibronectin levels as well as ROS were increased in diabetic OVE26 mice, and reduced in diabetic-EP1 null mice.20 Further confirming that antagonism of EP1 protects against PT injury.
Clinical Perspectives and Significance
In kidney disease, the PT is subjected to sodium and water transport challenges. Though basal PT water transport properties are not highly dependent on PGE2, a more pronounced role may be observed in kidney disease for example in diabetes, where the transport behavior of this segment is heightened. We have clearly shown that PGE2 acting on EP1 and EP4 receptors in MCT cells can modify growth responses and sodium transporter expression, and the EP1 receptor contributes to fibrosis and oxidative stress. Since PT growth promotes PT sodium hyper-reabsorption in chronic kidney disease, which in turn contributes to hyperfiltration and renal injury, PGE2 acting on PT EP receptors has the potential to influence PT sodium and water transport and injurious processes, and contribute to kidney disease.
References
Hao CM, Breyer MD . Physiological regulation of prostaglandins in the kidney. Annu Rev Physiol 2008;70;357–377.
Nasrallah R, Clark J, Hébert RL . Prostaglandins in the kidney: developments since Y2K. Clin Sci 2007;113;297–311.
Smyth EM, Grosser T, Wang M et al. Prostanoids in health and disease. J Lipid Res 2009;50 (Suppl):S423–S428.
Sauvant C, Hesse D, Holzinger H et al. Action of EGF and PGE2 on basolateral organic anion uptake in rabbit proximal renal tubules and hOAT1 expressed in human kidney epithelial cells. Am J Physiol 2004;286;F774–F783.
Sauvant C, Holzinger H, Gekle M . Short-term regulation of basolateral organic anion uptake in proximal tubular opossum kidney cells: prostaglandin E2 acts via receptor-mediated activation of protein kinase A. J Am Soc Nephrol 2003;14;3017–3026.
Sauvant C, Holzinger H, Gekle M . Prostaglandin E2 inhibits its own renal transport by downregulation of organic anion transporters rOAT1 and rOAT3. J Am Soc Nephrol 2006;17;46–53.
Syal A, Schiavi S, Chakravarty S et al. Fibroblast growth factor-23 increases mouse PGE2 production in vivo and in vitro. Am J Physiol 2006;290;F450–F455.
Herman MB, Rajkhowa T, Cutuli F et al. Regulation of renal proximal tubule Na+-K+-ATPase by prostaglandins. Am J Physiol 2010;298;F1222–F1234.
Lopes AG, Soares AC, Santos DP et al. PLA2/PGE2 are involved in the inhibitory effect of bradykinin on the angiotensin-(1-7)-stimulated Na(+-ATPase activity of the proximal tubule. Regul Pept 2004;117;37–41.
Vallon V . The proximal tubule in the pathophysiology of the diabetic kidney. Am J Physiol; 300;R1009–R1022.
Vallon V, Rose M, Gerasimova M et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol 2013;304;F156–F167.
Vallon V, Thomson SC . Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 2012;74;351–375.
Liu Y . Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 2006;69;213–217.
Ziyadeh FN . Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract 2008;82 (Suppl 1):S38–S41.
Mohamed R, Jayakumar C, Ranganathan PV et al. Kidney proximal tubular epithelial-specific overexpression of Netrin-1 suppresses inflammation and albuminuria through suppression of COX-2-mediated PGE2 production in streptozotocin-induced diabetic mice. Am J Pathol 2012;181;1991–2002.
Aoudjit L, Potapov A, Takano T . Prostaglandin E2 promotes cell survival of glomerular epithelial cells via the EP4 receptor. Am J Physiol 2006;290;F1534–F1542.
Vukicevic S, Simic P, Borovecki F et al. Role of EP2 and EP4 receptor-selective agonists of prostaglandin E(2) in acute and chronic kidney failure. Kidney Int 2006;70;1099–1106.
Yamamoto E, Izawa T, Juniantito V et al. Involvement of endogenous prostaglandin E2 in tubular epithelial regeneration through inhibition of apoptosis and epithelial-mesenchymal transition in cisplatin-induced rat renal lesions. Histol Histopathol 2010;25;995–1007.
Makino H, Tanaka I, Mukoyama M et al. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol 2002;13;1757–1765.
Thibodeau JF, Nasrallah R, He Y et al. PTGER1 deletion attenuates renal injury in diabetic mice. Am J Pathol 2013;183;1789–1802.
Haverty TP, Kelly CJ, Hines WH et al. Characterization of a renal tubular epithelial cell line which secretes the autologous target antigen of autoimmune experimental interstitial nephritis. J Cell Biol 1988;107;1359–1368.
Nasrallah R, Paris G, Hébert RL . Hypertonicity increases sodium transporters in cortical collecting duct cells independently of PGE2 . Biochem Biophys Res Commun 2012;418;372–377.
Nasrallah R, Xiong H, Hébert RL . Renal prostaglandin E2 receptor (EP) expression profile is altered in streptozotocin and B6-Ins2Akita type 1 diabetic mice. Am J Physiol 2007;292;278–284.
Burger D, Montezano AC, Nishigaki N et al. Endothelial microparticle formation by Angiotensin II Is mediated via Ang II receptor type I/NADPH oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol 2011;31;1898–1907.
Hébert RL, Carmosino M, Saito O et al. Characterization of a rabbit kidney prostaglandin F(2{alpha}) receptor exhibiting G(i)-restricted signaling that inhibits water absorption in the collecting duct. J Biol Chem 2005;280;35028–35037.
Hébert RL, Freedin D, Jacobson HR et al. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit collecting duct. Am J Physiol 1993;265;F643–F650.
Ladha MH, Lee KY, Upton TM et al. Regulation of exit from quiescence by p27 and Cyclin D1-CDK4. Mol Cell Biol 1998;18;6605–6615.
Zhuo JL, Li XC . Proximal nephron. Compr Physiol 2013;3;1079–1123.
Sedeek M, Callera GE, Montezano A et al. Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney—implications in type 2 diabetic nephropathy. Am J Physiol 2010;299;F1348–F1358.
Tang SCW, Lai KN . The pathogenic role of the renal proximal tubular cell in diabetic nephropathy 5. Nephrol Dial Transplant 2012;0;1–8.
Nasrallah R, Hassouneh R, Hébert RL . Chronic kidney disease: targeting prostaglandin E2 receptors. Am J Physiol 2014;307;F243–F250.
Nasrallah R, Landry A, Hébert RL . Characterization of the PGI2/IP system in cultured rat mesangial cells. Prostaglandins Leukot Essent Fatty Acids 2004;70;455–464.
Elberg D, Turman MA, Pullen N et al. Prostaglandin E2 stimulates cystogenesis through EP4 receptor in IMCD-3 cells. Prostaglandins Other Lipid Mediat 2012;98;11–16.
Frölich S, Olliges A, Kern N et al. Temporal expression of the PGE2 synthetic system in the kidney is associated with the time frame of renal developmental vulnerability to cyclooxygenase-2 inhibition. Am J Physiol 2012;303;F209–F219.
Li Z, Zhang Y, Kim WJ et al. PGE2 promotes renal carcinoma cell invasion through activated RalA. Oncogene 2013;32;1408–1415.
Liu Y, Rajagopal M, Lee K et al. Prostaglandin E2 mediates proliferation and chloride secretion in ADPKD cystic renal epithelia. Am J Physiol 2012;303;F1425–F1434.
Terada Y, Nakashima O, Inoshita S et al. TGF-β-activating kinase-1 inhibits cell cycle and expression of cyclin D1 and A in LLC-PK1 cells. Kidney Int 1999;56;1378–1390.
Datto MB, Li Y, Panus JF et al. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA 1995;92;5545–5549.
Hannon GJ, Beach D . p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 1994;371 (6494):257–261.
Iavarone A, Massagué J . Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-beta in cells lacking the CDK inhibitor p15. Nature 1997;387;417–422.
Massagué J . The transforming growth factor beta family. Annu Rev Cell Biol 1990;6;597–641.
Reynisdottir I, Polyak K, Iavarone A et al. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 1995;9;1831–1845.
Qian Q, Kassem KM, Beierwaltes WH et al. PGE2 causes mesangial cell hypertrophy and decreases expression of cyclin D3. Nephron Physiol 2009;113;7–14.
Franch HA, Shay JW, Alpern RJ et al. Involvement of pRB family in TGF beta-dependent epithelial cell hypertrophy. J Cell Biol 1995;129;245–254.
Zhang SL, To C, Chen X et al. Essential role(s) of the intrarenal renin-angiotensin system in transforming growth factor-beta1 gene expression and induction of hypertrophy of rat kidney proximal tubular cells in high glucose. J Am Soc Nephrol 2002;13;302–312.
Vervoort G, Veldman B, Berden JHM et al. Glomerular hyperfiltration in type I diabetes mellitus results from primary changes in proximal tubular sodium handling without changes in volume expansion. Eur J Clin Invest 2005;35;330–336.
Khan I . Antisense inhibition of cyclooxygenase-2 causes a selective suppression of the Na+-H+ exchanger isoform 3 in rat kidney in experimental colitis. Nephron 2002;91;120–128.
Segi E, Sugimoto Y, Yamasaki A et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun 1998;246;7–12.
Garvin JL . Angiotensin stimulates glucose and fluid absorption by rat proximal straight tubules. J Am Soc Nephrol 1990;1;272–277.
Schnermann J, Huang Y, Mizel D . Fluid reabsorption in proximal convoluted tubules of mice with gene deletions of claudin-2 and/or aquaporin. Am J Physiol 2013;305;F1352–F1364.
Lee SH, Ha SO, Koh HJ et al. Upregulation of cytosolic NADP+-dependent isocitrate dehydrogenase by hyperglycemia protects renal cells against oxidative stress. Mol Cells 2010;29;203–208.
Acknowledgements
This research was supported by, the Kidney Foundation of Canada and the Canadian Institutes for Health Research. DB is supported by a KRESCENT New Investigator Award. This research was supported by CIHR#220694 and KFoC#550826.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
In diabetes, the proximal tubule is subjected to sodium and water transport challenges. Prostaglandin E2, acting on prostaglandin receptors EP1 and EP4, modifies mouse proximal tubule transport properties and injurious responses. Since proximal tubule growth promotes sodium hyper-reabsorption, contributing to hyperfiltration and injury, proximal tubule prostaglandin receptors are promising therapeutic targets.
Rights and permissions
About this article
Cite this article
Nasrallah, R., Hassouneh, R., Zimpelmann, J. et al. Prostaglandin E2 increases proximal tubule fluid reabsorption, and modulates cultured proximal tubule cell responses via EP1 and EP4 receptors. Lab Invest 95, 1044–1055 (2015). https://doi.org/10.1038/labinvest.2015.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.79
This article is cited by
-
A Review of Prostanoid Receptors: Expression, Characterization, Regulation, and Mechanism of Action
Journal of Cell Communication and Signaling (2021)
-
PGE2 EP1 receptor inhibits vasopressin-dependent water reabsorption and sodium transport in mouse collecting duct
Laboratory Investigation (2018)