Abstract
To date, the role of Dickkopf 3 (Dkk3) on the pathogenesis of familial dilated cardiomyopathy (FDCM), and whether and how Dkk3 interferes with Wnt signaling in heart tissues remains unknown. Here, we demonstrate that strong Dkk3 expression was markedly downregulated in adult hearts from WT mice, and Dkk3 expression was upregulated suddenly in hearts from DCM mouse models. Using Dkk3 transgenic and knockout mice, as well as cTnTR141W transgenic mice, which manifests progressive chamber dilation and contractile dysfunction and has pathologic phenotypes similar to human DCM patients, we determined that transgenic expression of Dkk3 increased survival rate, improved cardiac morphology breakage and dysfunction, and ameliorated cardiac pathological changes in the cTnTR141W mice. In contrast, Dkk3 knockout reduced the survival rate and aggravated the pathological phenotypes of the cTnTR141W mice. The protective effects of Dkk3 appeared clearly at 3 months of age, peaked at 6 months of age, and decreased at 10 months of age in the cTnTR141W mice. Furthermore, we determined that Dkk3 upregulated Dvl1 (Dishevelled 1) and key proteins of the canonical Wnt pathway (cytoplasmic and nuclear β-catenin, c-Myc, and Axin2) and downregulated key proteins of the noncanonical Wnt pathway (c-Jun N-terminal kinase (JNK), Ca2+/calmodulin-dependent protein kinase II (CAMKII), and histone deacetylase 4 (HDAC4)). In contrast, Dkk3 knockout reversed these changes in the cTnTR141W mice. In summary, Dkk3 could prevent FDCM development in mice, especially in the compensatory stage, and probably through activation of the canonical and inhibition of the noncanonical Wnt pathway, which suggested that Dkk3 could serve as a therapeutic target for the treatment of cardiomyopathy and heart failure.
Similar content being viewed by others
Main
Dickkopf 3 (Dkk3) is a member of the Dickkopf family, which includes Dkk 1, 2, 4, and a unique Dkk3-related protein (DKKL1, or SGY-1). Dkk3 is abundant in the liver and brain, but is absent in the spleen and peripheral blood mononuclear cells.1 Dkk3 mediates potent antitumor effects, including reducing cell proliferation, anchorage-independent, growth and metastasis,2, 3, 4, 5, 6, 7 or inducing cancer cell apoptosis both in vitro and in murine tumor models.8, 9, 10 Furthermore, Dkk3 is upregulated during a series of disease and situation, including Alzheimer's disease,11 endochondral bone formation,12 retinal degeneration,13 and cellular senescence in prostate basal epithelial cells.14
Dkk3 is expressed in the developing and adult heart.15, 16 Dkk3 expression was increased in the afterloaded right ventricular (RV)17 and participated in aortic banding-induced cardiac hypertrophy via regulation of ASK-1-JNK/p38.18 Most of the knowledge about Dkk3 with regard to Wnt signaling comes from the study of cancers; Dkk3 can be an activator or an inhibitor of the canonical pathway,5, 8, 13, 19, 20, 21, 22, 23, 24 which is strictly context-dependent. However, whether and how Dkk3 interferes with Wnt signaling in the heart tissues has not been systematically examined thus far.
In the present study, we determined that Dkk3 expression increased significantly in both familial dilated cardiomyopathy mice (FDCM) model of cTnTR141W and adriamycin (ADR)-induced dilated cardiomyopathy mice model. We also determined how Dkk3 participates in the development of FDCM and whether Wnt signaling was involved using Dkk3 transgenic and knockout (ko) mice.
MATERIALS AND METHODS
Animals
The α-MHC-cTnTR141W (referred as cTnTR141W) FDCM transgenic mice was previously generated in our laboratory.25, 26, 27, 28 cDNA encoding mouse Dkk3 (GenBank accession no. NM_015814) was cloned into an expression plasmid under the α-MHC promoter and the heart-specific Dkk3 transgenic mouse (referred as Dkk3-ov) was generated by microinjection. Genotyping was performed by PCR analysis (forward, 5′-TCCTTCCCCGACGGTCACTT-3′ and reverse, 5′-CTGTCTCGGGTGCATAGCATCTG-3′) of genomic DNA under standard conditions, and target gene expression was analyzed by western blot analysis using antibody to Dkk3 (R&D). Dkk3-ko mice (129 background) were purchased from RIKEN (Japan) and crossed with C57BL/6J mice for six generations to obtain Dkk3-ko mice on the C57BL/6J background (referred as Dkk3-ko) in our laboratory. The Dkk3-ov mice were crossed with the cTnTR141W mice to generate α-MHC-cTnTR141W × Dkk3-ov double transgenic (referred as DTG-ov) mice. The Dkk3-ko mice were crossed with the cTnTR141W mice to generate α-MHC-cTnTR141W × Dkk3−/− (Dkk3 homozygote) mice (referred as DTG-ko).
All of the mice used in this study were maintained on a C57BL/6J genetic background and were bred in an AAALAC-accredited facility. The use of animals was approved by the Animal Care and Use Committees of The Institute of Laboratory Animal Science of Peking Union Medical College (ILAS-GC-2012-001).
Survival Analysis
The cumulative percent mortality in each mouse group was calculated every month, and the data from 1 to 10 months of age were summarized. Upon the death of each mouse, the body was autopsied by a pathologist, and morphological and pathological changes in the heart were recorded. Kaplan–Meier curves for survival analysis were compared using the log-rank test (SPSS 16.0 software).
Echocardiography
M-mode echocardiography was performed at 1, 3, 6, and 10 months of age on each mouse with the small animal echocardiography analysis system (Vevo770, Canada) as described previously.27, 28
Histological Analysis
For light microscopy, cardiac tissue from mice at 6 months of age was fixed in 4% formaldehyde and mounted in paraffin blocks, and sections were stained with H&E or Masson trichrome as described previously.27, 28 Myocytes were analyzed by an observer blinded to the mouse genotypes.
RNA Extraction, Quantification, and Real-time PCR
Mice were killed by cervical dislocation at 6 months of age, and total RNA was isolated from the heart using the TRIzol reagent (Invitrogen). First-strand cDNA was synthesized from 2 μg total RNA using random hexamer primers and Superscript III reverse transcriptase according to the manufacturer's protocol (Invitrogen). Procollagen type III α1 (Col3α1), natriuretic peptide type A (ANF), and natriuretic peptide type B (BNP) mRNA was detected by real-time PCR using GAPDH for normalization under standard conditions (primers: for Col3α1—forward, 5′-CTCAAGAGCGGAGAATACTGG-3′ and reverse, 5′-CAATGTCATAGGGTGCGATA-3′; for ANF—forward, 5′-ATGGGCTCCTTCTCCATCAC-3′ and reverse, 5′-TTATCTTCGGTACCGGAAGCTG-3′; for BNP—forward, 5′-ATGGATCTCCTGAAGGTGCTGTC-3′ and reverse, 5′-CTACAACAACTTCAGTGCGTTAC-3′; for GAPDH—forward, 5′-CAAGGTCATCCATGACAACTTTG-3′ and reverse, 5′-GTCCACCACCCTGTTGCTGTAG-3′).
Protein Extraction and Immunoblotting
Mice were killed by cervical dislocation, and total protein lysates from mouse heart were prepared as described previously.27, 28 After performing SDS-PAGE and transferring gels to nitrocellulose (Millipore), the membranes were incubated at 4 °C overnight with antibodies against Dkk3 (R&D), β-catenin (Abcam), c-Myc (Abcam), Axin2 (Abcam), Dvl1 (Abcam), phospho-CaMKII (Thr286) (Cell Signaling), CaMKII (Cell Signaling), phospho-HDAC4 (Ser632) (Cell Signaling), HDAC4 (Cell Signaling), phospho-SAPK/JNK (Thr183/Tyr185) (Cell Signaling), SAPK/JNK (Cell Signaling), phospho-protein kinase Cα/β (PKCα/β) (Thr638/641) (Cell Signaling), PKCα (Cell Signaling), phospho-PKCδ (Thr505) (Cell Signaling), and PKCδ (Cell Signaling). After incubation with the appropriate secondary antibodies for 1 h at room temperature, antibody binding was detected with HRP-conjugated immunoglobulin G (Santa Cruz) using a chemiluminescence detection system (Santa Cruz). Quantitative analysis of phosphorylated protein levels were normalized to the corresponding total protein, other proteins used GAPDH for normalization, and bands were quantified using the Image J software.
Dkk3-ko cell line
The Dkk3-ko cell line was established by CRISPR/Cas9. In brief, the paired synthesized oligonucleotide (Supplementary Table S1) for sgRNA targeting Dkk3 were annealed and cloned into the pGL3-U6-sgRNA-PGK-puromycin vector (no. 51133; Addgene, Cambridge, MA, USA). In accordance with the manufacturer’s instructions, H9c2 cells were transfected with sgRNA Dkk3 A and Dkk3 B (12 μg+12 μg) with 12 μg Cas9 plasmid by Lipofectamine 2000 (Invitrogen, Life Technologies, Carlsbad, CA, USA). After 24 h, blasticidine S hydrochloride (2 μg/ml; Life Tech, USA) and puromycin (1 μg/ml; Life Tech) was added. Genomic DNA of cell clones was extracted using phenol–chloroform and recovered by alcohol precipitation. Cell clones were genotyped by PCR (forward, 5′-GAAAGGGACGGGACTTGAAC-3′ and reverse, 5′-GGACTCTACTTCAGCACTCCTTTG-3′) and sequencing to detect the mutations. Cell clones with wanted mutation (referred as Dkk3-KO) were cultured for the subsequent experiments.
Luciferase assays
TOPFLASH (a firefly luciferase reporter plasmid, driven by two sets of three copies of the TCF binding site and herpes simplex virus thymidine kinase minimal promoter) and FOPFLASH (identical except for inactivating mutations of the TCF sites) were purchased from Merck Millipore, and pRL-cytomegalovirus (CMV; a constitutive, CMV-driven control, encoding Renilla luciferase) was from Promega. The expression construct for Dkk3 were generated by cloning full-length mouse Dkk3 cDNA fragment into the pCDNA3.1(+) vector (Invitrogen) and the presence of insert was verified using restriction digestion and DNA sequencing (refered as Dkk3-CMV). H9c2 cells cultured as described previously were transfected 1 day after plating by using Lipofectamine 2000. Transfections contained 0.5 μg of TOPFLASH or FOPFLASH plus 0.1 μg of pRL-CMV as the co-transfected control. After 6 h of incubation, the medium was replaced with normal culture medium. The luciferase reporter assay was performed in 24-well plates; 24 h after transfection, the cells were harvested, and luciferase activities were assayed by using the Dual-Luciferase system (Promega).
Immunofluorescence
H9c2 cells cultured as described previously3 were seeded onto 24-well plates containing round cover slides. The slides were first incubated with anti-β-catenin Ab (1:200; Abcam) overnight at 4 °C. Sections were washed with PBS and incubated with Alexa 488-conjugated goat anti-rabbit IgG (Invitrogen, USA) for 1 h at room temperature, and all slides were counterstained with 300 nM DAPI (4′,6-diamidino-2-phenylindole; Invitrogen). After washing with PBS, sections were mounted in ProLong Gold antifade reagent (Invitrogen). Images of the sections were collected and analyzed under confocal laser scanning microscopy (Leica TCS SP2, Germany).
Statistical Analysis
The data were analyzed with unpaired two-tailed Student’s t-tests for two groups, or one-way ANOVA for multiple groups followed by a Tukey’s post hoc analysis. The data were expressed as the means±s.d. from individual experiments. The differences were considered to be significant at P<0.05.
RESULTS
Dkk3 Expression Characteristics in Hearts of WT and DCM Mice Models
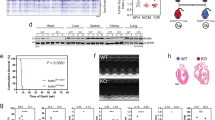
Dkk3 was strongly expressed in hearts of WT mice at embryonic day 16.5 to postnatal day 14, whereas its expression was obviously decreased thereafter with age (Figure 1a). Compared with decreased Dkk3 expression in adult hearts with age, Dkk3 expression was increased significantly in FDCM mice model of cTnTR141W and ADR-induced dilated cardiomyopathy mice model (Figures 1b and c, n=3, for establishment of ADR-induced DCM mice model please see Supplementary Methods). This phenomenon prompted us to further study the role of Dkk3 in cardiomyopathy pathogenesis.
Determination of Dickkopf 3 (Dkk3) expression and generation of Dkk3 overexpression transgenic mice. (a) Hearts were sampled from mice at embryonic 16.5 days, at birth, and at 3, 7, 14, 30, 60, and 90 days of age, and Dkk3 mRNA expression was detected by reverse transcription-PCR (RT-PCR). (b) Dkk3 expression in cTnTR141W transgenic mice and adriamycin (ADR)-induced mouse hearts. (c) Quantitative analysis of Dkk3 expression using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for normalization (n=3, ***P<0.001 versus wild-type (WT) mice). (d) The Dkk3 transgenic construct was generated by inserting the target genes under the control of an α-myosin heavy chain (α-MHC) cardiac-specific promoter and the transgenic mice were created following microinjection. (e) Two lines with Dkk3 overexpression were selected by western blot using GAPDH for normalization. (f) Quantitative analysis Dkk3 expression using GAPDH for normalization (n=3, ***P<0.001 versus non-transgenic (NTG) mice). HGH, human growth hormone.
We then generated Dkk3 transgenic mice overexpressing Dkk3 in the heart tissues and two lines of Dkk3 transgenic mice with high expression levels were selected from among 45 founders by western blotting (Figures 1d and f). The two Dkk3 transgenic mouse line were analyzed by M-mode echocardiography, which demonstrated thick-walled ventricles and smaller left ventricular diameters (Supplementary Table S2). These two lines displayed similar effects on the heart; we used one of them to mate with cTnTR141W mice and determined how Dkk3 participate in FDCM pathogenesis.
Dkk3 Increased the Survival Rate of cTnTR141W FDCM Transgenic Mice
Dkk3 transgenic mice and Dkk3-ko mice were crossed to the cTnTR141W mice as described in the Materials and Methods section and to obtain the NTG, Dkk3-ov, Dkk3-ko, cTnTR141W, DTG-ov, and DTG-ko mouse lines (Figure 2a), and the cumulative mouse mortality data from all of the six groups were recorded from 1 to 10 months of age (Figure 2b). Dilation and mural thrombi were observed in the mouse hearts during the post-mortem examinations. The survival rate was 100% in the NTG group (n=44) and Dkk3-ov group (n=32), whereas it was only 73.5% in the cTnTR141W group (n=49, P<0.001 versus NTG group) until 10 months of age. However, the survival rate was increased by 24.1% in the DTG-ov group (n=34, P<0.05 versus cTnTR141W group). Premature death occurred in the DTG-ko mice before 2 months of age, and the survival rate decreased by 30.1% in the DTG-ko group until 10 months of age (n=37, P<0.05 versus cTnTR141W group). Transgenic expression of Dkk3 increased, whereas Dkk3-ko reduced the survival rate of cTnTR141W mice.
Genotyping and survival analysis. (a) Genotyping by genomic PCR for cTnTR141W (370 bp PCR products), Dickkopf 3 (Dkk3) transgenic (480 bp PCR products), and Dkk3-kockout (ko) (946 bp PCR products). (b) Cumulative percent mortality for non-transgenic mice (NTG) (n=44), Dkk3-ov (n=32), Dkk3-ko (n=36), cTnTR141W (n=49, ***P<0.001 versus NTG group), DTG-ov (n=34, #P<0.05 versus cTnTR141W), and DTG-ko (n=37, #P<0.05 versus cTnTR141W) was calculated every month from 1 to 10 months of age.
Dkk3 Improves Cardiac Morphology Breakage and Dysfunction in cTnTR141W FDCM Transgenic Mice
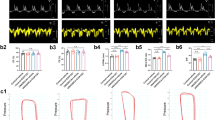
To confirm the protective effect of Dkk3 on FDCM, we analyzed the cardiac geometry and function with M-mode echocardiography in all of the six groups at 1, 3, 6, and 10 months of age. The cTnTR141W transgenic mice displayed typical FDCM phenotypes with dilated chambers, thin walls, and cardiac dysfunction. However, the FDCM phenotypes were significantly ameliorated owing to the transgenic expression of Dkk3 in the cTnTR141W mice, demonstrated by the 10.6% reduction in left ventricular diameter at end systole (LVESD) (Figure 3a, P<0.001) and 18.6% increase in left ventricular posterior wall thickness at end systole (LVPWS) (Figure 3b, P<0.001), which lead to a 32.9% increase in left ventricular percent fractional shortening (LVFS) (Figure 3c, P<0.01). In contrast, the FDCM phenotypes were significantly exacerbated in Dkk3-ko cTnTR141W mice, as demonstrated by the 9.2% increase in LVESD (Figure 3a, P<0.05), 12.0% reduced LVPWS (Figures 3b, P<0.05), and 18.7% reduced LVFS (Figure 3c, P<0.05). The protective effect of Dkk3 on FDCM development was observed obviously from 3 months of age and peaked at 6 months of age, but the protective effect was decreased at the end-stage DCM at 10 months of age (Figures 3d and f and Supplementary Tables S3–S6).
Dickkopf 3 (Dkk3) ameliorated cardiac geometrical and functional phenotypes in cTnTR141W transgenic mice. Echocardiographic parameters of left ventricular (LV) diameter at end systole (LVESD) (a), LV posterior wall thickness at end systole (LVPWS) (b), and LV percent fractional shortening (LVFS) (c) were analyzed in non-transgenic (NTG), Dkk3-ov, Dkk3-ko, cTnTR141W, DTG-ov, and DTG-ko mice at 6 months of age (*P<0.05 versus NTG mice; **P<0.01 versus NTG mice; #P<0.05 versus cTnTR141W mice; ##P<0.01 versus cTnTR141W mice; ###P<0.001 versus cTnTR141W mice). Echocardiographic parameters of LVESD (d), LVPWS (e), and LVFS (f) were analyzed in the cTnTR141W, DTG-ov, and DTG-ko mice at 1, 3, 6, and 10 months of age. Ko, Knockout.
Dkk3 Ameliorates Cardiac Pathological Changes in cTnTR141W FDCM Transgenic Mice
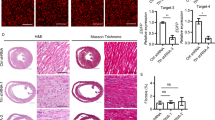
Heart from the all the six groups were sampled for gross morphology examination and histological examination at 6 months of age (Figure 4). Pathological phenotypes of dilated chambers, thin walls, myocytes disarray, and fibrosis in the cTnTR141W mice were significantly reduced because of the transgenic expression in DTG-ov mice. In contrast, Dkk3-ko increased the heart to body weight ratio, malalignment and collagen accumulation in the interstitial space, and hypertrophic marker (ANF and BNP) expression significantly in the DTG-ko mice compared with that in the cTnTR141W mice.
Dickkopf 3 (Dkk3) ameliorated pathological changes in the cTnTR141W transgenic mice. (a) Heart-weight (HW) to body-weight (BW) ratio was determined for non-transgenic (NTG) (n=11), Dkk3-ov (n=7), Dkk3-ko (n=10, *P<0.05 versus NTG group), cTnTR141W (n=8), and DTG-ov (n=8), DTG-ko (n=10, #P<0.05 versus cTnTR141W) transgenic mice. (b) Hematoxylin and eosin (H&E) staining patterns of the whole-heart longitudinal sections from mice at 6 months of age. (c) Magnification of H&E-stained sections of left ventricle (magnification × 400, scan bar is 20 μm), demonstrating disparate pathological changes. (d) Magnification of Masson trichrome-stained left ventricle section (magnification × 400); myocytes are stained red; collagenous tissue is stained green. The expression of procollagen type III α1 (Col3α1) (e), natriuretic peptide type A (ANF) (f), and natriuretic peptide type B (BNP) (g) were detected by real-time PCR using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) for normalization (n=3, #P<0.05 versus cTnTR141W). Ko, Knockout.
Taken together, echocardiography and histological examination consistently demonstrated that Dkk3 overexpression could improve and Dkk3-ko exacerbate the pathological phenotypes of FDCM in mice.
Dkk3 Activates Canonical Wnt/β-catenin Pathway in cTnTR141W FDCM Transgenic Mice
Dvl is a key factor of the Wnt pathway. Corresponding with the increase in Dkk3, Dvl1 levels increased significantly in the cTnTR141W mice (Figures 5a and b, n=3). In contrast, Dvl1 level was reduced with Dkk3-ko in the cTnTR141W mice (Figures 5a and b, n=3).
Dickkopf 3 ((Dkk3) activates the canonical Wnt pathway in vivo and in vitro. (a) Dishevelled 1 (Dvl1), cytoplasmic and in nuclear β-catenin, Axin2, and c-Myc expression was measured by western blot in the non-transgenic (NTG), Dkk3-ov, Dkk3-ko, cTnTR141W, DTG-ov, and DTG-ko transgenic mouse hearts at 6 months of age. The quantitative analysis of Dvl1 (b), cytoplasmic β- (c), nuclear β-catenin (d), Axin2 (e), and c-Myc (f) using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) o histone H3 for normalization (n=3, *P <0.05 versus NTG mice; **P<0.01 versus NTG mice; #P<0.05 versus cTnTR141W mice; ##P<0.01 versus cTnTR141W mice). (g) H9c2 cells were transfected with pTOPFlash or pFOPFlash (inactive, mutant T-cell factor (TCF) sites) along with pRL-CMV. Luciferase activity of vehicle-treated, pFOPFlash-transfected cells of wild-type (WT) was set at 1, and other results are expressed as fold activation relative to that value (n=3, *P<0.05, **P<0.01 vs WT cells). (h) Immunodetection of β-catenin in H9c2 cells. β-Catenin staining appeared green showing the distrabution of β-catenin protein and the sections were counterstained blue with DAPI (4',6-diamidino-2-phenylindole) to visualize the nuclei. CMV, cytomegalovirus; Ko, Knockout.
Furthermore, we detected the activation state of the canonical Wnt/β-catenin pathway, corresponding with increased Dkk3 expression, and cytoplasmic and nuclear β-catenin level (Figures 5a, c, and d, n=3) were increased. In line, the Axin2 (Figures 5a and e, n=3) and c-Myc (Figures 5a and f, n=3) were subsequently increased in the DTG-ov mice. In contrast, after Dkk3-ko, cytoplasmic and nuclear β-catenin level (Figure 5a, c and d, n=3) were decreased, and Axin2 and c-Myc expressions (Figures 5a, e, and f, n=3) were subsequently reduced in the DTG-ko mice. And to confirm the activation of Dkk3 on the canonical Wnt pathway, Dkk3-ko and overexpression in H9c2 cells were established, and then were transfected with pTOPFlash or pFOPFlash (inactive, mutant TCF sites), a β-catenin report system, along with pRL-CMV. We found that Dkk3 also increased the β-catenin activation in vitro (Figure 5g, n=3). Furthermore, the activation of Dkk3 on β-catenin were confirmed by immunofluorescence (Figure 5h). These results suggested that canonical Wnt/β-catenin signaling was activated by Dkk3 overexpression and inhibited by Dkk3-ko in vivo and in vitro.
Dkk3 Inhibits the Wnt/Ca2+ and Wnt/JNK Pathway in cTnTR141W FDCM Transgenic Mice
The noncanonical Wnt pathway divides into three major branches that are mediated through JNKs, PKC, and CAMKII. We detected the three major branches of the noncanonical Wnt pathway in all of the six groups. The JNK signaling and CAMKII signaling were inhibited with the transgenic Dkk3 expression (Figures 6a and c, n=3). Furthermore, HDAC4 phosphorylation was subsequently inhibited corresponding with inactivation of CAMKII signaling (Figures 6a and d, n=3).
Dickkopf 3 (Dkk3) inhibited the noncanonical Wnt pathway in cTnTR141W familial dilated cardiomyopathy (FDCM) transgenic mice. (a) Phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185), total JNK, phospho-Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Thr286), total CaMKII, phospho-histone deacetylase 4 (HDAC4) (Ser632), and total HDAC4 expression were measured by western blot in non-transgenic (NTG), Dkk3-ov, Dkk3-ko, cTnTR141W, DTG-ov, and DTG-ko transgenic mouse hearts at 6 months of age. Quantitative analysis of phospho-JNK (b), phospho-CaMKII (c), and phospho-HDAC4 (d) total JNK, total CaMKII, and total HDAC4 were used for normalization, respectively (n=3, *P<0.05 versus NTG mice; #P<0.05 versus cTnTR141W mice). Ko, knockout.
In contrast, JNK and CAMKII signaling were significantly activated (Figures 6a and d, n=3), and HDAC4 phosphorylation was subsequently increased (Figures 6a and d, n=3) with Dkk3-ko. However, Dkk3 overexpression and knockout did not alter PKCδ and PKCα phosphorylation in the mouse hearts (Supplementary Figure S1). These results suggested that Dkk3 inhibited JNK and CAMKII signaling in the noncanonical Wnt pathway in mouse hearts.
The protective effect of Dkk3 on FDCM development peaked at 6 months of age, but decreased at the end-stage DCM at 10 months of age (Figure 3d and f and Supplementary Tables S3–S6). Therefore, we also detected the proteins involved in the canonical and noncanonical Wnt pathway using 10 months of age; we found that Dkk3 activate canonical Wnt/β-catenin pathway and inhibit the Wnt/Ca2+ and Wnt/JNK pathway at 10 months of age (Figure 7), but the regulation on those pathway were weaker than that of the 6 months of age, and these results were consistent with the protective effect of Dkk3 on FDCM phenotypic development by echocardiograph analysis.
The expression of proteins in the canonical Wnt pathway (a), including Dishevelled 1 (Dvl1), cytoplasmic and in nuclear β-catenin, Axin2, and c-Myc, and proteins in the noncanonical Wnt pathway (b), including phospho-c-Jun N-terminal kinase (JNK) (Thr183/Tyr185), total JNK, phospho-Ca2+/calmodulin-dependent protein kinase II (CaMKII) (Thr286), total CaMKII, phospho-histone deacetylase 4 (HDAC4) (Ser632), and total HDAC4 were measured by western blot in the heart tissues of non-transgenic (NTG), Dkk3-ov, Dkk3-ko, cTnTR141W, DTG-ov, and DTG-ko transgenic mice at 10 months of age. Dkk3, Dickkopf 3; Ko, knockout.
DISCUSSION
Dkk3 is expressed in the developing and adult heart.15, 16 Our results demonstrated that strong Dkk3 expression was markedly downregulated in WT adult mouse heart, whereas upregulated suddenly in DCM mouse heart (Figure 1). This result is consistent with the report that Dkk3 expression is increased in the afterloaded RV.17 However, it found that Dkk3 expression was downregulated in the left ventricle myocardial samples from DCM patients in end-stage heart failure.18 These results suggest that Dkk3 could be upregulated in compensation stage and downregulated in the decompensation stage of heart failure.
It will be meaningful to understand the function of Dkk3 on cardiomyopathy pathogenesis using a model with a progressive pathology development. The cTnTR141W transgenic mouse manifested progressive chamber dilation and contractile dysfunction and has pathologic phenotypes similar to human DCM patients.25, 27 We used the cTnTR141W transgenic mouse, the Dkk3 transgenic mice and the Dkk3-ko mice to analyze Dkk3 function on the pathological development of DCM. The results revealed that transgenic Dkk3 expression increased survival rate, improved cardiac morphology breakage and dysfunction and ameliorated cardiac pathological changes in the cTnTR141W FDCM mice. In contrast, Dkk3-ko reduced cTnTR141W FDCM mouse survival rate and aggravated their pathological phenotypes (Figures 2). However, the protective effects of Dkk3 appeared obviously at 3 months of age, peaked at 6 months of age, and decreased at 10 months of age in the cTnTR141W FDCM mice (Figures 3d and f and Supplementary Tables S3–S6). These data suggested that Dkk3 could have a protective role mainly in the compensation stage of heart failure.
Most of the knowledge about Dkk3 on Wnt signaling came from studying cancers, and can activate14 or inhibit the canonical Wnt pathway,5, 8, 13, 19, 20, 21, 22, 23, 24 which appears to be strictly context-dependent, including cell surface receptors and coexpressed ligands. However, the precise mechanism by which Dkk3 interferes with Wnt signaling in heart tissues was unclear, although it has been observed that Dkk3 participated in the aortic banding-induced cardiac hypertrophy via ASK-1-JNK/p38 pathway regulation.18
Wnt signaling principally involves canonical (β-catenin-dependent) and noncanonical (β-catenin-independent) signaling pathways, which are divided into three major branches that are mediated through JNKs, PKC, and Ca2+/CAMKII. We assessed changes in both the canonical and noncanonical Wnt pathways. We determined that Dkk3 upregulated Dvl1 and key proteins of the canonical Wnt pathway (cytoplasmic and nuclear β-catenin, Axin2, and c-Myc) (Figures 5 and 8) and downregulated key proteins of the noncanonical Wnt pathway (JNK, CAMKII, and HDAC4) (Figures 6 and 8), whereas Dkk3 did not obviously change the PKC branch (Supplementary Figure S1). In contrast, these effects on those branches were reversed by Dkk3-ko in the cTnTR141W transgenic mice. And, the activation of Dkk3 on the canonical Wnt pathway was also confirmed in vitro (Figure 5). The protective effect of Dkk3 on FDCM development peaked at 6 months of age, but decreased at the end-stage DCM at 10 months of age (Figures 3d–f and Supplementary Tables S3–S6). Therefore, we also detected the proteins involved in canonical and noncanonical Wnt pathway at 10 months of age. We found that Dkk3 activate canonical Wnt/β-catenin pathway and inhibit the Wnt/Ca2+ and Wnt/JNK pathway at 10 months of age, but the regulation on those pathway were weaker than that of the 6 months of age (Figures 5), and these results were consistent with the phenotypic development by echocardiograph analysis.
Wnt signaling is critical for proper myocardial differentiation but is silenced in normal fully differentiated myocardium;29, 30, 31, 32 however, the pathway becomes reactivated in disease, including hypertrophy.33 Dvl is a positive regulator of the Wnt pathway,34 and cardiac-specific Dvl1 led to severe cardiomyopathy.33 Spontaneous cardiac hypertrophy was observed in β-catenin-depleted mice, and an attenuation of cardiac hypertrophy was observed in mice with stabilized β-catenin.35 However, the importance of β-catenin-dependent signaling for the development of cardiac hypertrophy has been confirmed in others reports.36, 37, 38, 39 The noncanonical pathways are also involved in hypertrophy. JNK activation produces a fibrotic/failing heart phenotype in vivo and in vitro.40, 41 Dominant-negative JNK prevents pressure overload hypertrophy in rats.42 CaMKII inhibition can attenuate cardiac hypertrophy,43 and CAMKII phosphorylation was associated with increased histone deacetylase 4 phosphorylation, which has been associated with cardiac hypertrophy.44 Canonical and noncanonical Wnt signaling are antergic in some systems,31, 45 which raises the interesting possibility that those pathways are actually part of a large Wnt signaling network in which a unique combination of effectors are activated in a cell-type-dependent manner.
Taken together, the amelioration of FDCM by Dkk3 may be partly through canonical Wnt pathway activation and noncanonical Wnt pathway inhibition.
DCM is a primary heart muscle disease,46 and despite recent advances in pharmacological and surgical therapies, disability and morbidity due to DCM remain common.47, 48, 49 Therefore, screening and investigation of important modifier genes involved in the cardiomyopathy pathogenesis is still a major concern in the heart disease field. Elevated Dkk3 levels are specifically associated with AD and might serve as a potential noninvasive AD biomarker in the plasma;14 moreover, evidences accumulated indicated that Dkk3 is both a potential tumor biomarker and effective anticancer agent.50, 51 In view of our findings, Dkk3 could be a potential target for cardiomyopathy treatment.
Our data provide compelling evidence that Dkk3 can prevent the FDCM development in mice, especially in the compensatory stage, probably through activation of the canonical and inhibition of the noncanonical Wnt pathway (especially the Wnt/Ca2+ and the Wnt/JNK branches), which suggests that Dkk3 is a therapeutic target for cardiomyopathy and heart failure treatment.
Accession codes
References
Zhang K, Watanabe M, Kashiwakura Y et al. Expression pattern of REIC/Dkk-3 in various cell types and the implications of the soluble form in prostatic acinar development. Int J Oncol 2010;37:1495–1501.
Abarzua F, Sakaguchi M, Takaishi M et al. Adenovirus-mediated overexpression of REIC/Dkk-3 selectively induces apoptosis in human prostate cancer cells through activation of c-Jun-NH2-kinase. Cancer Res 2005;65:9617–9622.
Gu YM, Ma YH, Zhao WG et al. Dickkopf3 overexpression inhibits pancreatic cancer cell growth in vitro. World J Gastroenterol 2011;17:3810–3817.
Koppen A, Ait-Aissa R, Koster J et al. Dickkopf-3 expression is a marker for neuroblastic tumor maturation and is down-regulated by MYCN. Int J Cancer 2008;122:1455–1464.
Mizobuchi Y, Matsuzaki K, Kuwayama K et al. REIC/Dkk-3 induces cell death in human malignant glioma. Neuro Oncol 2008;10:244–253.
Than SS, Kataoka K, Sakaguchi M et al. Intraperitoneal administration of an adenovirus vector carrying REIC/Dkk-3 suppresses peritoneal dissemination of scirrhous gastric carcinoma. Oncol Rep 2011;25:989–995.
Tsuji T, Nozaki I, Miyazaki M et al. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non-small-cell lung carcinomas. Biochem Biophys Res Commun 2001;289:257–263.
Dellinger TH, Planutis K, Jandial DD et al. Expression of the Wnt antagonist Dickkopf-3 is associated with prognostic clinicopathologic characteristics and impairs proliferation and invasion in endometrial cancer. Gynecol Oncol 2012;126:259–267.
Ueno K, Hirata H, Majid S et al. Wnt antagonist DICKKOPF-3 (Dkk-3) induces apoptosis in human renal cell carcinoma. Mol Carcinogen 2011;50:449–457.
Yang ZR, Dong WG, Lei XF et al. Overexpression of Dickkopf-3 induces apoptosis through mitochondrial pathway in human colon cancer. World J Gastroenterol 2012;18:1590–1601.
Zenzmaier C, Marksteiner J, Kiefer A et al. Dkk-3 is elevated in CSF and plasma of Alzheimer's disease patients. J Neurochem 2009;110:653–661.
Aslan H, Ravid-Amir O, Clancy BM et al. Advanced molecular profiling in vivo detects novel function of dickkopf-3 in the regulation of bone formation. J Bone Miner Res 2006;21:1935–1945.
Nakamura RE, Hunter DD, Yi H et al. Identification of two novel activities of the Wnt signaling regulator Dickkopf 3 and characterization of its expression in the mouse retina. BMC Cell Biol 2007;8:52.
Zenzmaier C, Sklepos L, Berger P . Increase of Dkk-3 blood plasma levels in the elderly. Exp Gerontol 2008;43:867–870.
Krupnik VE, Sharp JD, Jiang C et al. Functional and structural diversity of the human Dickkopf gene family. Gene 1999;238:301–313.
Monaghan AP, Kioschis P, Wu W et al. Dickkopf genes are co-ordinately expressed in mesodermal lineages. Mech Dev 1999;87:45–56.
Urashima T, Zhao M, Wagner R et al. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol 2008;295:H1351–H1368.
Zhang Y, Liu Y, Zhu XH et al. Dickkopf-3 attenuates pressure overload-induced cardiac remodelling. Cardiovasc Res 2014;102:35–45.
Caricasole A, Ferraro T, Iacovelli L et al. Functional characterization of WNT7A signaling in PC12 cells: interaction with A FZD5 x LRP6 receptor complex and modulation by Dickkopf proteins. J Biol Chem 2003;278:37024–37031.
Hoang BH, Kubo T, Healey JH et al. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res 2004;64:2734–2739.
Kawano Y, Kitaoka M, Hamada Y et al. Regulation of prostate cell growth and morphogenesis by Dickkopf-3. Oncogene 2006;25:6528–6537.
Lee EJ, Jo M, Rho SB et al. Dkk3, downregulated in cervical cancer, functions as a negative regulator of beta-catenin. Int J Cancer 2009;124:287–297.
Wang XY, Yin Y, Yuan H et al. Musashi1 modulates mammary progenitor cell expansion through proliferin-mediated activation of the Wnt and Notch pathways. Mol Cell Biol 2008;28:3589–3599.
Yue W, Sun Q, Dacic S et al. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis 2008;29:84–92.
Juan F, Wei D, Xiongzhi Q et al. The changes of the cardiac structure and function in cTnTR141W transgenic mice. Int J Cardiol 2008;128:83–90.
Lu D, Dong W, Zhang X et al. WIF1 causes dysfunction of heart in transgenic mice. Transgenic Res 2013;22:1179–1189.
Lu D, Ma Y, Zhang W et al. Knockdown of cytochrome P450 2E1 inhibits oxidative stress and apoptosis in the cTnT(R141W) dilated cardiomyopathy transgenic mice. Hypertension 2012;60:81–89.
Lu D, Zhang L, Bao D et al. Calponin 1 inhibits dilated cardiomyopathy development in mice through the ɛPKC pathway. Int J Cardiol 2014;173:146–153.
Cingolani OH . Cardiac hypertrophy and the Wnt/Frizzled pathway. Hypertension 2007;49:427–428.
Oka T, Xu J, Molkentin JD . Re-employment of developmental transcription factors in adult heart disease. Semin Cell Dev Biol 2007;18:117–131.
Schneider VA, Mercola M . Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 2001;15:304–315.
Tzahor E, Lassar AB . Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev 2001;15:255–260.
Malekar P, Hagenmueller M, Anyanwu A et al. Wnt signaling is critical for maladaptive cardiachypertrophy and accelerates myocardial remodeling. Hypertension 2010;55:939–945.
Wallingford JB, Habas R . The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development 2005;132:4421–4436.
Baurand A, Zelarayan L, Betney R et al. β-Catenin downregulation is required for adaptive cardiac remodeling. Circ Res 2007;100:1353–1362.
Chen X, Shevtsov SP, Hsich E et al. The β-catenin/T-cell factor/lymphocyte enhancer factor signaling pathway is required for normal and stress-induced cardiac hypertrophy. Mol Cell Biol 2006;26:4462–4473.
Hahn JY, Cho HJ, Bae JW et al. β-Catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts. J Biol Chem 2006;281:30979–30989.
Qu J, Zhou J, Yi XP et al. Cardiac-specific haploinsufficiency of β-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol 2007;43:319–326.
van de Schans VA, van den Borne SW, Strzelecka AE et al. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension 2007;49:473–480.
Petrich BG, Eloff BC, Lerner DL et al. Targeted activation of c-Jun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects. J Biol Chem 2004;279:15330–15338.
Wang Y, Su B, Sah VP et al. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem 1998;273:5423–5426.
Choukroun G, Hajjar R, Fry S et al. Regulation of cardiac hypertrophy in vivo by the stress-activated protein kinases/c-Jun NH2-terminal kinases. J Clin Invest 1999;104:391–398.
Diedrichs H, Chi M, Boelck B et al. Increased regulatory activity of the calcineurin/NFAT pathway in human heart failure. Eur J Heart Fail 2004;6:3–9.
Haberland M, Montgomery RL, Olson EN . The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 2009;10:32–42.
Pandur P, Lasche M, Eisenberg LM et al. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 2002;418:636–641.
Richardson P, McKenna W, Bristow M et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996;93:841–842.
Ho KK, Anderson KM, Kannel WB et al. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993;88:107–115.
Jessup M, Brozena S . Heart failure. N Engl J Med 2003;348:2007–2018.
Petretta M, Pirozzi F, Sasso L et al. Review and metaanalysis of the frequency of familial dilated cardiomyopathy. Am J Cardiol 2011;108:1171–1176.
Mühlmann G, Untergasser G, Zitt M et al. Immunohistochemically detectable dickkopf-3 expression in tumor vessels predicts survival in gastric cancer. Virchows Arch 2010;456:635–646.
Veeck J, Dahl E . Targeting the Wnt pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys Acta 2012;1825:18–28.
Acknowledgements
This research was supported, in part, by Beijing Natural Science Foundation (7122111) and National Natural Science Foundation (31301932).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Laboratory Investigation website
This study demonstrates a new role for Dkk3 in mouse models of familial dilated cardiomyopathy development. Activation of Wnt/β-catenin is seen in the compensatory stage, as well as inhibition of the non-canonical Wnt pathway, which suggested that Dkk3 may serve as a therapeutic target for the treatment of cardiomyopathy and heart failure.
Supplementary information
Rights and permissions
About this article
Cite this article
Lu, D., Bao, D., Dong, W. et al. Dkk3 prevents familial dilated cardiomyopathy development through Wnt pathway. Lab Invest 96, 239–248 (2016). https://doi.org/10.1038/labinvest.2015.145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2015.145
This article is cited by
-
The emerging plasma biomarker Dickkopf-3 (DKK3) and its association with renal and cardiovascular disease in the general population
Scientific Reports (2021)
-
Transcriptome analysis of genes related to gonad differentiation and development in Muscovy ducks
BMC Genomics (2020)
-
DKK3 attenuates JNK and AP-1 induced inflammation via Kremen-1 and DVL-1 in mice following intracerebral hemorrhage
Journal of Neuroinflammation (2020)
-
Wnt5a is associated with right ventricular dysfunction and adverse outcome in dilated cardiomyopathy
Scientific Reports (2017)
-
Differential effects on lung and bone metastasis of breast cancer by Wnt signalling inhibitor DKK1
Nature Cell Biology (2017)