Abstract
Population studies suggest putative links between vitamin D (VD)-deficiency and risk of cancer and diabetes. The insulin/IGF-I receptor represents a signaling target of the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) that is implicated in both diabetes and cancer, therefore we hypothesized that VD actions may be mediated through this adhesion molecule. In this study, we show that 1,25 vitamin D3 and its analogues EB1089 and KH1060 potently inhibit CEACAM1 expression in cancer cells. This effect was associated with significant reductions in mRNA and protein levels, resulting from transcriptional and posttranslational actions respectively. Insulin/IGF-I-mediated IRS-1 and Akt activation were enhanced by VD treatment. Similarly, CEACAM1 downregulation significantly upregulated the insulin and IGF-I receptors and mimicked the effect of VD-mediated enhanced insulin/IGF-I receptor signaling. Despite improved insulin/IGF-I signaling, the anti-proliferative actions of VD were preserved in the absence or presence of forced CEACAM1 expression. Forced CEACAM1, however, abrogated the anti-invasive actions of VD. Our findings highlight CEACAM1 as a target of VD action. The resulting inhibition of CEACAM1 has potentially beneficial effects on metabolic disorders without necessarily compromising the anticancer properties of this vitamin.
Similar content being viewed by others
Main
In addition to regulation of calcium homeostasis, 1,25 dihydroxyvitamin D3 (VD) and its low calciomimetic analogues have important roles in the regulation of cell proliferation, differentiation and apoptosis. These include inhibition of growth of breast, prostate, colon, thyroid and pituitary neoplastic cells.1, 2, 3, 4 VD exerts its functions by binding to the VD receptor (VDR), which forms heterodimers with retinoid X receptors (RXRs) in the presence of ligand. VDR/RXR heterodimers bind VD response elements (VDREs) in the promoters of target genes. We have previously reported that VD compounds promote accumulation of the cyclin-dependent kinase inhibitor p27 through PTEN-dependent and -independent pathways,3 resulting in diminished neoplastic growth and reduced metastatic spread in an orthotopic mouse model of thyroid cancer.5 Furthermore, VD enhances cell adhesion through the extracellular matrix protein fibronectin, further limiting cell proliferation and metastasis.6
VD compounds have also been recognized for their favorable therapeutic effects in the treatment of diabetes.7, 8, 9 The mechanisms underlying these actions, however, remain poorly understood. In this report, we examine the effects of VD on the carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) that we propose may be a mediator of both insulin action and tumorigenesis.
CEACAM1 is a transmembrane protein member of the CEA superfamily10 that is widely expressed in hematopoietic, epithelial and endothelial cells in which it has a key role in the control of immunity,11 cell adhesion10 and angiogenesis.12 CEACAM1 shows homophilic adhesion as well as heterophilic binding to other CEA family members in vitro13 and is believed to be important in the maintenance of normal tissue architecture. Importantly, CEACAM1 is also a substrate of the insulin receptor (IR). IR-mediated phosphorylation of CEACAM114 results in binding and sequestration of the Shc adaptor protein,15, 16 thus potentially limiting insulin signaling. Additionally, CEACAM1 modulates hepatic insulin clearance.16, 17
The role of this adhesive factor in cancer remains controversial. Some studies described it to be downregulated in breast,18, 19 colorectal,20, 21 prostate,22, 23 bladder,24 endometrial25 and hepatocellular carcinomas.26 Forced transfection of human prostate cancer cells with CEACAM1 results in significantly lower growth rates and reduced tumorigenesis.27 Further, introduction of CEACAM1 into human prostate cancer cells significantly suppresses tumor growth in mouse xenografts.23 These observations suggest that CEACAM1 acts as a tumor suppressor. In contrast, however, CEACAM1 is overexpressed in primary lung cancer,28, 29 gastric adenocarcinoma30 and malignant melanoma.31 This upregulation is associated with malignant progression and metastasis, suggesting that CEACAM1 can also act as an oncogenic signal. In a previous study, we attempted to reconcile these seemingly contradictory data by dissecting the proliferative effects from metastatic functions of this adhesion molecule. We showed that forced expression of CEACAM1-inhibited cell proliferation and tumor growth whereas CEACAM1 silencing accelerated cell cycle progression and promoted tumor growth in mouse xenografts,32 consistent with the tumor-suppressive actions previously reported; in contrast, forced expression of CEACAM1 increased cell migration and invasion, providing an explanation for upregulation of CEACAM1 associated with metastatic spread. Consistent with these results, we identified upregulation of CEACAM1 predominantly in small thyroid carcinomas with lymph node metastasis.32
The effect of VD on CEACAM1 has not been previously reported. In this study, we used CEACAM1-expressing and -deficient cells as a model to investigate the effects of VD compounds on the regulation of this adhesion molecule and its putative actions on the insulin/IGF-I receptors and their associated functions.
MATERIALS AND METHODS
Cell Lines and Cell Culture
Human WRO and MRO thyroid cancer cells and ARO colon cancer cell lines were obtained from Dr J Fagin (Memorial Sloan-Kettering Cancer Center, New York, NY, USA)33 and maintained in RPMI 1640 supplemented with 10% FBS, 2 mmol/l L-glutamine, 1 mmol/l sodium pyruvate and 1 × non-essential amino acid (Sigma-Aldrich, Irvine, UK). The cells were cultured in a standard humidified incubator at 37 °C in a 5% CO2 atmosphere. Cell viability was assessed using Trypan blue exclusion before all experiments. Insulin (300 nM) and IGF-I (13 nM) treatments were performed for 10 min in serum-free conditions. Dose and treatment durations were based on earlier time and dose-response studies.
Downregulation and Forced Expression of CEACAM1
MRO cells express endogenous CEACAM1, therefore clones with knockdown of CEACAM1 (pSilencing-CEACAM1 siRNA) or its scrambled vector as a negative control were selected with G418 (1 mg/ml) as described previously.32 WRO cells do not express detectable endogenous CEACAM1, therefore clones were stably transfected to express CEACAM1 using the pcDNA 3.1/Zeo-L-CEACAM1 expression vector or the empty vector as a control, followed by ZEOCIN (0.125 μg/ml, Invitrogen, Carlsbad, CA, USA) selection.
VD and Compounds
1α, 25-Dihydroxyvitamin D3 (herein referred to as 1,25 VD) and its analogues EB1089 and KH1060 (22, 24-diene-24a, 26a, 27a-trihomo-1α, 25-dihydroxyvitamin D3) were provided by Dr L Binderup of LEO Pharmaceuticals (Ballerup, Denmark). To determine VD effects on CEACAM1 expression, cells were synchronized in serum-free medium for 24 h followed by VD, EB1089 or KH1060 treatment (10−7 M) for another 72 h in 10% charcoal-treated serum growth medium. These conditions were based on earlier time-course and dose-response experiments demonstrating the efficacy of VD compounds.3, 6 To interrupt protein synthesis, cells were pretreated with cycloheximide (CHX) (50 μg/ml) for 48 and 72 h.
Protein Isolation and Western Blotting
Cells were lysed in a lysis buffer (0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40 and 1 × PBS) containing proteinase inhibitors (100 μg/ml phenylmethylsulfonyl fluoride, 13.8 μg/ml aprotinin (Sigma, St Louis, MO, USA) and 1 mM sodium orthovanadate. Total cell lysates were incubated on ice for 30 min, followed by micro-centrifugation at 10 000 g for 10 min at 4 °C. Protein concentrations of the supernatants were determined by the Bio-Rad protein assay.
Equal amounts of protein (50 μg) were mixed with 2 × sodium dodecyl sulfate (SDS) sample buffer, boiled for 4 min and separated by 10 or 12% SDS-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes (Bio-Rad Laboratories). Nonspecific binding was blocked with 5% non-fat milk in 1 × TBST (Tris-buffered saline with 0.1% Tween-20). Primary antibodies were directed against CEACAM1 (4D1/C2, kindly provided by C Wagener, Hamburg),32 phospho-IR pY1158/1162/1163 in the kinase activation loop and phospho-IR pY972, which is the juxtamembrane site for IRS1 adaptor recruitment (both 1:1000, Biosource, Camarillo, CA, USA), IR-β (1:1000, Upstate, Lake Placid, NY, USA), phospho-IRS1 pY612, which is a key site of IRS1 activation (1:1000, Invitrogen, Camarillo, CA, USA), IRS1 (0.5 μg/ml, Upstate), phospho-Shc that represents the main site of Grb recruitment (1:1000, Cell Signaling Technology, Beverely, MA, USA), phospho-Akt pS473 (1:1000, Cell Signaling, Akt (1:1000, Cell Signaling Technology) or actin (1:1000, Sigma, Oakville, Ontario, Canada). After washing three times, each for 10 min in 1 × TBST, blots were exposed to the secondary antibody (anti-mouse or anti-rabbit IgG-HRP, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:2000 and visualized using ECL chemiluminescence detection system (Amersham, Buckinghamshire, UK). Band intensities were quantified by scanning densitometry. All studies were performed in triplicate experiments.
Preparation of Cell Pellets for Immunohistochemistry
Cells were washed three times in cold Ca2+-Mg2+-free D-PBS, gently scraped and centrifuged into pellets. The pellets were fixed in 10% formalin for 1–3 h and coated in 2% bactoagar until solidified, then fixed in 10% formalin and embedded in paraffin. Sections of 4 μm thickness were de-waxed in five changes of xylene and rehydrated through graded alcohols into water. Sections were microwave heated in 10 mM citrate buffer at pH 6.0 inside a pressure cooker. Endogenous peroxidase and biotin activities were blocked respectively using 3% hydrogen peroxide and Vector's avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA, USA). Sections were treated for 10 min with protein blocker (ID Labs London, Ontario, Canada), then incubated overnight with anti-CEACAM1 mouse monoclonal antibody (4D1/C2) at 1:20032 followed by 30 min each with biotinylated linking reagent (ID Labs) and HRP-conjugated Ultra Streptavidin Labeling Reagent (ID Labs). Color development was performed using freshly prepared NovaRed solution (Vector Labs) and counterstained with Mayer's hematoxylin. Stained sections were dehydrated through graded alcohols, cleared in xylene and mounted in Permount (Fisher, Ontario, Canada).
RNA Extraction, RT-PCR and Real-Time PCR
Total RNA was isolated using RNeasy kit (QIAGEN, Mississauga, Ontario, Canada), followed by DNAse I treatment. Six hundred nanograms of total RNA were reverse transcribed in 30 μl reaction mixture containing 500 μM each of deoxynucleotide triphosphate, 12U ribonuclease inhibitor and MultiScribe reverse transcriptase using TaqMan reverse transcription reagents (Applied Biosystems, Branchburg, NJ, USA). The reaction mixture was incubated at 25 °C for 10 min, 48 °C for 30 min and 95 °C for 5 min. The PCR primers for CEACAM1 or the house-keeping gene PGK-1 and the PCR conditions were previously described.32 Real-time PCR was performed on cDNA samples in triplicate using an ABI PRISM 7900HT sequence detection system (Applied Biosystems). SYBR Green PCR Master Mix (AB Applied Biosystems) was used according to the manufacturer's protocol. The primers for amplification of human CEACAM1-L were used as described.34 As an endogenous control, the primers for human 18S ribosomal RNA were used to normalize for variations in RNA. After optimization, PCR reactions were performed in a 20 μl volume containing 0.5 μM primers and 1 μl cDNA under the following conditions: 50 °C for 2 min, 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The results were analyzed using a comparative method. The amount of target, normalized to the endogenous reference, is given by the formula 2−ΔΔCt where Ct represents the threshold cycle, indicating the fractional cycle number at which the amount of amplified target reaches a fixed threshold. All experiments were performed in triplicate.
Transfection and Luciferase Assays
Human CEACAM1 promoter-pGL3 constructs and empty vector were used as described previously.35 Cells were plated into six-well plates (0.15 × 106 cells per well) and transfected on the following day with 5 μl per well LipofectAMINE and 2 μg of DNA per well. Transfection efficiency was monitored by simultaneous co-transfection with a β-galactosidase reporter construct (0.2 μg per well). After 24 h, the cells were treated with vehicle and 1,25 VD (10−7 M) for another 24 h before the cells were lysed in buffer (Promega, Madison, WI, USA). Luciferase activity was measured in a luminometer. β-Galactosidase activity was measured to normalize for variations in transfection efficiency. Promoter activity of each construct was expressed as firefly luciferase/β-galactosidase activity. Each experiment was performed in triplicate and repeated on three occasions.
Cell Proliferation and Invasion
Cell cycle analysis was used to determine cell proliferation. Cells were synchronized in serum-free medium for 24 h, then after 72 h of 1,25 VD treatment, the cells were trypsinized, washed with D-PBS (Ca2+-Mg2+-free) and fixed with cold 80% ethanol for 1 h on ice. The fixed cells were washed with staining buffer (0.2% Triton X-100 and 1 mM/l EDTA, pH 8.0, in PBS) and re-suspended in the staining buffer containing 50 μg/ml DNase-free RNase A (Sigma) and 50 μg/ml propidium iodide for 1 h. Cell cycle analysis was done by flow cytometry (Becton Dickinson, San Jose, CA, USA) using Cellquest analysis. The result was analyzed using the Modfit DNA Analysis program (Verity Software House, Topsham, ME, USA). Direct cell counts were also performed using a Beckman Coulter counter (Fullerton, CA, USA). Cell invasion was performed using a Matrigel system as previously described.32
Statistical Analysis
Data are presented as mean±s.e. Differences were assessed by Student's paired t-test. Significance level was assigned at P<0.05.
RESULTS
1,25 Vitamin D3 Inhibits CEACAM1 Expression in Human Carcinoma Cells
We have previously shown abundant endogenous expression of CEACAM1 in MRO and ARO but not in WRO cells.32 To investigate the effects of VD compounds on CEACAM1 expression, ARO and MRO cells were treated with 1,25 VD and its non-hypercalcemic compounds EB1089 and KH1060. The dose of 10−7 M and duration of 72-h treatment were based on earlier dose- and time-course studies demonstrating the functional activity of these compounds in these cells.3 Here we show that VD compounds dramatically inhibit CEACAM1 expression. This was shown in MRO and ARO cells as determined by western blotting (Figure 1a) and also by immunohistochemistry of cell pellets (Figure 1b). WRO cells do not endogenously express detectable CEACAM1 and there was no change after exposure to VD compounds (data not shown).
1,25-Vitamin D3 (VD) and its analogues potently inhibit CEACAM1 expression in human carcinoma cells. (a) Human MRO and ARO cells treated for 72 h with VD and two of its non-calciomimetic analogues, EB1089 and KH1060 (all at 10−7 M for 72 h) show significantly reduced CEACAM1 protein expression by western blotting and by immunohistochemistry of MRO cell pellets (b) as indicated.
1,25 Vitamin D3 Inhibits CEACAM1 through Multiple Mechanisms
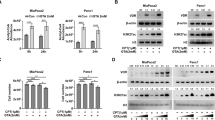
To investigate the mechanism(s) of VD compound inhibition of CEACAM1 expression, MRO cells were treated with 1,25 VD for 72 h and subjected to RT-PCR for CEACAM1 mRNA (Figure 2a). This demonstrated a marked reduction of mRNA levels, which was confirmed by quantitative real-time PCR (Figure 2b). To examine whether 1,25 VD has inhibitory effects on CEACAM1 gene transcription, we performed luciferase reporter assays using deletional fragments of the human CEACAM1 promoter (Figure 2c). Inhibition was noted with the proximal −369-bp promoter fragment and the 2.6-kb fragment, consistent with one VDRE in the −369-bp fragment (−238 to −258) and six VDRE sites in the larger fragment (−238/−258, −534/−548, −1342/−1356, −1440/−1454, −2282/−2296 and −2565/−2578). However, the degree of promoter attenuation was not sufficiently impressive to explain the marked effect on protein expression. Thus, we examined the effect of the protein synthesis inhibitor CHX on 1,25 VD action at 48 and 72 h. The effect of CHX mimicked VD compound action, and the combination of VD and CHX displayed no additive effects (Figure 2d), consistent with impact of both compounds on the post-transcriptional inhibition of CEACAM1.
1,25-Vitamin D3 (VD) inhibits CEACAM1 through multiple mechanisms. To investigate the mechanisms of VD inhibition of CEACAM1, MRO cells were treated with VD at 10−7 M for 72 h. (a) Examination of mRNA by RT-PCR shows a reduction in transcripts in cells incubated with 1,25 VD; PGK in lower panels serves as a control. (b) Quantitative real-time PCR examination confirms significant inhibition of mRNA levels by 1,25 VD in triplicate experiments; * indicates statistical significance (P=0.001). (c) To verify that 1,25 VD inhibits CEACAM1 gene transcription, MRO cells were transfected with the indicated fragments of the human CEACAM1 promoter in the absence or presence of 1,25 VD. Luciferase reporter activity demonstrates significant inhibition with both the −2.6 and −369-bp fragments (P=0.004); the effects are consistent in proportion with the known single VDRE in the −369-bp fragment and six VDRE sites in the larger fragment. Data presented are the mean luciferase activity adjusted for β-galactosidase activity (±s.e.) compared with control wells of three independent transfections. (d) To identify a post-transcriptional mechanism for CEACAM1 inhibition, 1,25 VD-treated cells were examined in the absence or presence of the protein synthesis inhibitor cycloheximide (CHX) 50 μg/ml for 48 and 72 h. CHX inhibits CEACAM1 at both time periods (48 h shown) in a manner mimicking VD treatment and is not additive to it.
VD-Mediated CEACAM1 Inhibition Modifies Insulin/IGF-1 Receptor Signaling
To investigate the signaling consequences of VD-mediated CEACAM1 inhibition, insulin/IGF-I receptor activation was examined in cells with or without stable CEACAM1 downregulation (Figure 3a). All VD treatments involved 72 h of exposure to the vitamin under steroid-depleted conditions. First, we noted that stable knockdown of CEACAM1 resulted in a nearly twofold increase in IR levels (Figure 3b; left panels). Similarly, IGF-I receptor levels were induced with the forced reduction of CEACAM1 (Figure 3b; right panels). To study the impact of the increases in insulin and IGF-I receptor levels in the face of CEACAM1 knockdown, we compared the signaling ability of these ligands in the presence or absence of VD. In control cells, insulin-induced activation of its immediate substrate IRS-1 (Figure 3c; left panel) and consequently promoted Akt phosphorylation (Figure 3d; left panel). This effect was preserved in the absence or presence of VD (Figure 3c; left panels). The presence of VD, however, augmented the effects of IGF-I on IRS1 phosphorylation (Figure 3c; right panels). Consistent with this observation, Akt activation in response to IGF-I was also more pronounced in the presence of VD (Figure 3d; right panels). Similarly, forced silencing of CEACAM1 resulted in recapitulation of the effects of VD with enhanced IRS1 tyrosyl and Akt serine phosphorylation (Figures 3c and d). It is noteworthy that these insulin and IGF-I responses in WRO cells that are endogenously devoid of CEACAM1 were not influenced by the presence or absence of 1,25 VD (data not shown). Taken together, these findings indicate that CEACAM1 inhibition by VD promotes insulin/IGF-I signaling in a manner qualitatively analogous to the impact of forced CEACAM1 downregulation.
1,25-Vitamin D3 inhibition or CEACAM1 silencing enhances insulin and IGF-1 signaling. (a) The signaling consequences of vitamin D-mediated CEACAM1 inhibition, insulin (left panels) and IGF-I (right panels) actions were examined in MRO cells downregulated for CEACAM1 (siRNA) in the absence (C) or presence of VD. All VD treatments involved 72 h of exposure to the 1,25 vitamin D3(10−7 M) as detailed under Materials and Methods. (b) CEACAM1 knockdown results in significant upregulation of insulin receptor (left panels) and IGF-I receptor (right panels) levels. (c) In scrambled control cells, insulin induces activation of the immediate substrate IRS-1 to enhance Akt phosphoryation (d). VD treatment enhances insulin/IGF-I signaling evidenced by augmentation of their effects on IRS1/Akt activation. Similarly, CEACAM1-downregulated cells (shRNA) demonstrate enhanced insulin/IGF-I actions. Results shown are representative of three independent experiments, each performed in triplicate. Densitometric means of these experiments are shown in the corresponding bar graphs, and significant differences are shown with P-values.
Anti-Proliferative Actions of VD are Sustained Despite Enhanced Insulin/IGF-1 Receptor Signaling
Given the ability of CEACAM1 to inhibit cell growth,32 the suppressive effect of VD compounds on CEACAM1 with resulting enhancement of insulin/IGF-I signaling could potentially attenuate VD anti-neoplastic actions. To specifically address this possibility, we examined the anti-proliferative actions of VD in the presence or absence of forced CEACAM1 expression. Cell cycle progression was monitored by flow cytometry in WRO cells forced to express CEACAM1. These studies revealed that 1,25 VD and its two analogues, EB1089 and KH 1060 significantly induce G0/G1 arrest (Figure 4a) and decrease S-phase entry (Figure 4b) and G2 residence (Figure 4c). Furthermore, direct cell counting confirmed the preserved ability of VD compounds to reduce cell proliferation in the absence or presence of forced CEACAM1 expression (Figure 4d). These findings indicate that the anti-proliferative actions of 1,25 VD compounds are not impaired in the presence of forced CEACAM1 expression.
Impact of 1,25-vitamin D3 on cell proliferation and invasion in the absence or presence of forced CEACAM1 expression. (a–c) Cell cycle progression was monitored by flow cytometry (FACS) in cells forced to express CEACAM1 and their empty-vector controls as indicated. 1,25 vitamin D3 (10−7 M) significantly induces G0/G1 arrest, decreases S-phase entry without altering G2/M phase residence. These actions were preserved in the absence or presence of forced CEACAM1 expression. (d) Direct cell counting confirms reduction of cell number by VD treatment in control as well as cells forced to express CEACAM1. The VD anti-proliferative actions are preserved in the absence or presence of forced CEACAM1 expression. (e) Cells overexpressing CEACAM1 and control WRO cells were treated with vehicle or VD for 72 h, the cells were trypsinized and 2 × 104 cells were plated in a Matrigel insert with the same treatment for another 24 h. The invasive cells in the whole membrane were scanned and counted by Aperio software imaging analysis. Student's t-test from two independent experiments demonstrates a statistically significant impact of CEACAM1 that abrogates the effect of VD on cell invasion.
To investigate the influence of CEACAM1 on the known VD-induced inhibitory effects on cell invasion, cells with forced CEACAM1 expression and control WRO cells were treated with vehicle or VD in a Matrigel assay. This approach identified a statistically significant impact of CEACAM1 in abrogating the inhibitory effect of VD on cell invasion (Figure 4e).
DISCUSSION
VD deficiency has been shown to predispose individuals to diabetes. Administration of VD or its analogues protects against the development of type 1 diabetes in non-obese diabetes-prone mice, principally by modulating the immune response.7, 36, 37 In humans, increased VD intake early in life may reduce the risk of type 1 diabetes.38 Conversely, reduced VD intake and circulating concentrations are associated with reduced insulin sensitivity and an increased risk of type 2 diabetes and the metabolic syndrome.39, 40 VDR polymorphisms have also been associated with type 2 diabetes.41 Although the exact mechanisms of VD action in type 2 diabetes are unclear, it is generally accepted that stimulation of insulin synthesis and secretion represent important actions.
To explore alternate mechanisms for VD in the regulation of insulin/IGF-I action, we examined the effects of this vitamin on CEACAM1, a putative target of the IR. CEACAM1 is generally regarded as a negative regulator of insulin action. Several mechanisms have been suggested, including sequestration of the Shc adaptor protein.15 Binding to Shc, CEACAM1 competes with the insulin and its related IGF-I receptor for IRS-1 recruitment. Thus, the presence of CEACAM1 would be expected to impair Akt activation a point of common downstream signaling for the insulin and IGF-I receptors. Thus, to investigate the putative role of CEACAM1 in VD regulation of insulin/IGF-I-mediated actions, we examined signaling of these growth factors in the absence or presence of CEACAM1 silencing. Here we provide an additional mechanism for the negative impact of CEACAM1 insulin/IGF-I signaling. Specifically, we show that forced CEACAM1 silencing significantly increases insulin and IGF-I receptor levels. Consistent with this observation, CEACAM1 downregulation markedly enhances IRS1 phosphorylation to promote Akt activation in response to insulin/IGF-I. Similarly, VD-mediated CEACAM1 inhibition resulted in augmented IRS-1 phosphorylation, particularly in response to IGF-I. Consistent with the integrative effect of signaling through the insulin and IGF-I receptors, Akt activation was augmented in response to both ligands in the presence of VD. These findings provide further functional evidence for the impact of VD-mediated inhibition of CEACAM1 in modulating insulin/IGF-I action.
In a previous study, we showed that forced expression of CEACAM1-inhibited cell proliferation and tumor growth.32 Conversely, we have shown that CEACAM1 silencing in MRO cells accelerates cell cycle progression and promotes tumor growth in mouse xenografts.32 As such, CEACAM1 inhibition by VD might have been expected to interfere with the anti-proliferative actions of the vitamin. Indeed, along with others, we3, 42 have previously demonstrated the ability of VD and its non-hypercalcemic analogue EB1089 or 22-oxacalciltriol to inhibit human carcinoma cell proliferation through multiple mechanisms. These include cell cycle arrest associated with upregulation of p27 and downregulation of Skp2.4 In an orthotopic mouse xenograft model, we showed that systemic administration of VD significantly inhibited WRO tumor growth and metastasis.5 In this study, we found that VD and its analogues EB1089 and KH1060 significantly reduce CEACAM1 expression, which prompted us to explore the role of CEACAM1 in mediating VD effects on insulin and IGF-I action.
The mechanisms and mediators involved in the regulation of CEACAM1 have not been well defined. TPA and the calcium ionophore A23187 increase CEACAM1 expression in human endometrial carcinoma cells.35 IFN-γ induces CEACAM1 mRNA and protein levels in human colon carcinoma cells34 while androgens enhance CEACAM1 promoter activity,43 and all-trans-RA and 9-cis-RA induce CEACAM1 expression in human monoblast U937 cells.44 Other than an inhibitory effect by the Sp2 transcription factor,45 little is known about signals that diminish CEACAM1 expression.
To investigate the mechanisms of VD inhibition of CEACAM1 in cancer cells, we examined the effects of VD on CEACAM1 transcription. 1,25 VD significantly reduced CEACAM1 mRNA expression as shown by RT-PCR, quantitative real-time PCR and promoter reporter assays. These findings are consistent with the results of in silico screening that identified five putative VDREs in the CEACAM1 promoter.46 However, the effects on transcription noted in this study were not sufficient in magnitude to explain the dramatic reduction in CEACAM1 protein levels. Indeed, the effect of CHX was similar to VD compounds, and the combination of both did not show additive effects at multiple time points compared with either alone. Furthermore, given the relatively long protein half-life of CEACAM1 of 26 h,47 our findings based on 48- and 72-h incubations are consistent with VD inhibition of CEACAM1 at both the transcriptional and post-transcriptional levels.
On the basis of our previous observation that CEACAM1 inhibits cell proliferation and tumor growth,32 and our present finding that VD reduces CEACAM1 expression, we asked whether VD growth inhibitory actions are altered by CEACAM1. We chose MRO and WRO cells because of their distinct patterns of CEACAM1 expression. We found that VD compounds inhibit MRO cell proliferation by inducing G0/1 cell cycle arrest, which was associated with upregulation of p27 and inhibition of Rb phosphorylation. To investigate the role of CEACAM1 in VD-induced action in the regulation of cell proliferation, WRO cells with forced CEACAM1 expression were selected. Significantly, 1,25 VD-inhibited cell proliferation in both cell lines as determined by cell cycle analysis and cell counts. These growth inhibitory effects did not show measurable difference despite differing CEACAM1 levels. These findings suggest that CEACAM1 does not interfere with VD anti-proliferative actions. Given the ability of CEACAM1 to promote metastatic spread,32 inhibition of this adhesive factor by 1,25 VD shown here likely represents a more relevant mechanism for this hormone's metabolic actions. These findings are consistent with our earlier observations that VD is effective in limiting WRO thyroid cancer metastasis formation using an orthotopic mouse model.6
In summary, this study demonstrates the ability of VD to potently inhibit CEACAM1, resulting in enhanced insulin/IGF-I-mediated action. Through gain- and loss-of-function approaches, we demonstrate that such potentially favorable metabolic signals can be mediated through CEACAM1 inhibition. However, downregulation of CEACAM1 with enhancement of insulin/IGF-I receptor signaling did not adversely interfere with the ability of VD compounds to inhibit neoplastic cell growth. This latter effect is consistent with VD's pleiotropic properties. How VD targeting of CEACAM1 can influence metastasis development requires further study. Nevertheless, our data highlight the potential therapeutic role of VD through CEACAM1 inhibition in metabolic diseases without necessarily compromising the risk of cancer progression.
References
Holick MF . Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol 2006;92:49–59.
Lamprecht SA, Lipkin M . Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer 2003;3:601–614.
Liu W, Asa SL, Fantus IG, et al. Vitamin D arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through PTEN/Akt-dependent and -independent pathways. Am J Pathol 2002;160:511–519.
Liu W, Asa SL, Ezzat S . Vitamin D and its analog EB1089 induce p27 accumulation and diminish association of p27 with Skp2 independent of PTEN in pituitary corticotroph cells. Brain Pathol 2002;12:412–419.
Dackiw AP, Ezzat S, Huang P, et al. Vitamin D3 administration induces nuclear p27 accumulation, restores differentiation, and reduces tumor burden in a mouse model of metastatic follicular thyroid cancer. Endocrinology 2004;145:5840–5846.
Liu W, Asa SL, Ezzat S . 1{alpha},25-Dihydroxyvitamin D3 targets PTEN-dependent fibronectin expression to restore thyroid cancer cell adhesiveness. Mol Endocrinol 2005;19:2349–2357.
Mathieu C, Gysemans C, Giulietti A, et al. Vitamin D and diabetes. Diabetologia 2005;48:1247–1257.
Tai K, Need AG, Horowitz M, et al. Vitamin D, glucose, insulin, and insulin sensitivity. Nutrition 2008;24:279–285.
Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, et al. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10:185–197.
Obrink B . CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol 1997;9:616–626.
Gray-Owen SD, Blumberg RS . CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 2006;6:433–446.
Ergun S, Kilik N, Ziegeler G, et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell 2000;5:311–320.
Kinugasa T, Kuroki M, Takeo H, et al. Expression of four CEA family antigens (CEA, NCA, BGP and CGM2) in normal and cancerous gastric epithelial cells: up-regulation of BGP and CGM2 in carcinomas. Int J Cancer 1998;76:148–153.
Fournes B, Sadekova S, Turbide C, et al. The CEACAM1-L Ser503 residue is crucial for inhibition of colon cancer cell tumorigenicity. Oncogene 2001;20:219–230.
Poy MN, Ruch RJ, Fernstrom MA, et al. Shc and CEACAM1 interact to regulate the mitogenic action of insulin. J Biol Chem 2002;277:1076–1084.
Poy MN, Yang Y, Rezaei K, et al. CEACAM1 regulates insulin clearance in liver. Nat Genet 2002;30:270–276.
Deangelis AM, Heinrich G, Dai T, et al. Carcinoembryonic antigen-related cell adhesion molecule 1: a link between insulin and lipid metabolism. Diabetes 2008;57:2296–2303.
Riethdorf L, Lisboa BW, Henkel U, et al. Differential expression of CD66a (BGP), a cell adhesion molecule of the carcinoembryonic antigen family, in benign, premalignant, and malignant lesions of the human mammary gland. J Histochem Cytochem 1997;45:957–963.
Plunkett TA, Ellis PA . CEACAM1: a marker with a difference or more of the same? J Clin Oncol 2002;20:4273–4275.
Neumaier M, Paululat S, Chan A, et al. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA 1993;90:10744–10748.
Brummer J, Neumaier M, Gopfert C, et al. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene 1995;11:1649–1655.
Kleinerman DI, Troncoso P, Lin SH, et al. Consistent expression of an epithelial cell adhesion molecule (C-CAM) during human prostate development and loss of expression in prostate cancer: implication as a tumor suppressor. Cancer Res 1995;55:1215–1220.
Luo W, Tapolsky M, Earley K, et al. Tumor-suppressive activity of CD66a in prostate cancer. Cancer Gene Ther 1999;6:313–321.
Kleinerman DI, Dinney CP, Zhang WW, et al. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res 1996;56:3431–3435.
Bamberger AM, Riethdorf L, Nollau P, et al. Dysregulated expression of CD66a (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am J Pathol 1998;152:1401–1406.
Takanishi K, Miyazaki M, Ohtsuka M, et al. Inverse relationship between P-glycoprotein expression and its proliferative activity in hepatocellular carcinoma. Oncology 1997;54:231–237.
Hsieh JT, Luo W, Song W, et al. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res 1995;55:190–197.
Ohwada A, Takahashi H, Nagaoka I, et al. Biliary glycoprotein mRNA expression is increased in primary lung cancer, especially in squamous cell carcinoma. Am J Respir Cell Mol Biol 1994;11:214–220.
Wang L, Lin SH, Wu WG, et al. C-CAM1, a candidate tumor suppressor gene, is abnormally expressed in primary lung cancers. Clin Cancer Res 2000;6:2988–2993.
Kinugasa T, Kuroki M, Takeo H, et al. Expression of four CEA family antigens (CEA, NCA, BGP and CGM2) in normal and cancerous gastric epithelial cells: up-regulation of BGP and CGM2 in carcinomas. Int J Cancer 1998;76:148–153.
Thies A, Moll I, Berger J, et al. CEACAM1 expression in cutaneous malignant melanoma predicts the development of metastatic disease. J Clin Oncol 2002;20:2530–2536.
Liu W, Wei W, Winer D, et al. CEACAM1 impedes thyroid cancer growth but promotes invasiveness: a putative mechanism for early metastases. Oncogene 2007;26:2747–2758.
Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab 2008;93:4331–4341.
Fahlgren A, Baranov V, Frangsmyr L, et al. Interferon-gamma tempers the expression of carcinoembryonic antigen family molecules in human colon cells: a possible role in innate mucosal defence. Scand J Immunol 2003;58:628–641.
Bamberger AM, Briese J, Gotze J, et al. Stimulation of CEACAM1 expression by 12-O-tetradecanoylphorbol-13-acetate (TPA) and calcium ionophore A23187 in endometrial carcinoma cells. Carcinogenesis 2006;27:483–490.
Mathieu C, Laureys J, Sobis H, et al. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992;41:1491–1495.
Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002;51:1367–1374.
Hypponen E, Laara E, Reunanen A, et al. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 2001;358:1500–1503.
Scragg R, Sowers M, Bell C . Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care 2004;27:2813–2818.
Ford ES, Ajani UA, McGuire LC, et al. Concentrations of serum vitamin D and the metabolic syndrome among US adults. Diabetes Care 2005;28:1228–1230.
Reis AF, Hauache OM, Velho G . Vitamin D endocrine system and the genetic susceptibility to diabetes, obesity and vascular disease. A review of evidence. Diabetes Metab 2005;31:318–325.
Suzuki S, Takenoshita S, Furukawa H, et al. Antineoplastic activity of 1,25(OH)2D3 and its analogue 22-oxacalcitriol against human anaplastic thyroid carcinoma cell lines in vitro. Int J Mol Med 1999;4:611–614.
Phan D, Sui X, Chen DT, et al. Androgen regulation of the cell-cell adhesion molecule-1 (Ceacam1) gene. Mol Cell Endocrinol 2001;184:115–123.
Botling J, Oberg F, Nilsson K . CD49f (alpha 6 integrin) and CD66a (BGP) are specifically induced by retinoids during human monocytic differentiation. Leukemia 1995;9:2034–2041.
Phan D, Cheng CJ, Galfione M, et al. Identification of Sp2 as a transcriptional repressor of carcinoembryonic antigen-related cell adhesion molecule 1 in tumorigenesis. Cancer Res 2004;64:3072–3078.
Sinkkonen L, Malinen M, Saavalainen K, et al. Regulation of the human cyclin C gene via multiple vitamin D3-responsive regions in its promoter. Nucleic Acids Res 2005;33:2440–2451.
Horstkorte R, Lee HY, Lucka L, et al. Biochemical engineering of the side chain of sialic acids increases the biological stability of the highly sialylated cell adhesion molecule CEACAM1. Biochem Biophys Res Commun 2001;283:31–35.
Acknowledgements
This work was supported by the Rita Banach Thyroid Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, W., Guo, M., Ezzat, S. et al. Vitamin D inhibits CEACAM1 to promote insulin/IGF-I receptor signaling without compromising anti-proliferative action. Lab Invest 91, 147–156 (2011). https://doi.org/10.1038/labinvest.2010.144
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.2010.144