Abstract
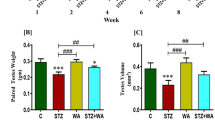
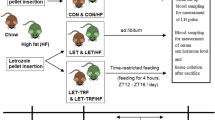
Goto-Kakizaki rats are an important model of type 2 diabetes, but it is difficult to maintain and expand colonies of these rats because they exhibit poor fertility. In this work, we studied the estrous cycle of Goto-Kakizaki and Wistar rats to characterize differences that might underlie these reproductive difficulties. We monitored rats from weaning to vaginal opening to assess pubertal development, and we monitored the estrous cycle and basal glycemia of each rat for 20 days at 1 month of age and at 6 months of age. At an early age we found no differences between Goto-Kakizaki and Wistar rats with respect to the onset of puberty or the periodicity of the estrous cycle. However, at 6 months Goto-Kakizaki rats spent more time in proestrus and less time in estrus each cycle, compared to Wistar rats. This delay in proceeding from proestrus to estrus could reflect a dysregulation of the hypothalamic-pituitary-gonadal axis, accompanying progression of the diabetic condition. It might also cause anovulatory cycles, which could explain the reduced reproductive capacity of Goto-Kakizaki rats.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
World Health Organization. Diabetes Fact sheet no. 312, http://www.who.int/mediacentre/factsheets/fs312/en/. Accessed May 10, 2015.
Mayo Clinic. Diseases and Conditions: Diabetes, http://www.mayoclinic.org/diseases-conditions/diabetes/basics/definition/con-20033091. Accessed May 10, 2015.
Lechuga-Sancho, A.M. et al. Reduction in the number of astrocytes and their projections is associated with increased synaptic protein density in the hypothalamus of poorly controlled diabetic rats. Endocrinology 147, 5314–5324 (2006).
Nandy, A. et al. The effect of insulin signaling on female reproductive function independent of adiposity and hyperglycemia. Endocrinology. 151, 1863–1871 (2010).
Distiller, L.A., Sagel, J. & Morley, J.E. Pituitary responsiveness to luteinizing hormone-releasing hormone in insulin-dependent diabetes mellitus. Diabetes 24, 378–380 (1975).
Livshits, A. & Seidman, D.S. Fertility issues in women with diabetes. Womens Health (Lond. Engl.) 5, 702–707 (2009).
Akash, M. & Chen, K. Goto-Kakizaki rats: Its suitability as non-obese diabetic animal model for spontaneous type 2 diabetes mellitus. Curr. Diabetes Rev. 9, 1–10 (2013).
Díaz, J. & Balibrea, J. Modelos animales de intolerancia a la glucosa y diabetes tipo 2. Nutr. Hosp. 22, 160–168 (2007).
Anzalone, C., Hong, L., Lu, J. & Lapolt, P. Influences of age and ovarian follicular reserve on estrous cycle patterns, ovulation, and hormone secretion in the long-evans rat. Biol. Reprod. 64, 1056–1062 (2001).
Rivest, R.W. Sexual maturation in female rats: hereditary, developmental and environmental aspects. Experientia 47, 1027–1038 (1991).
Maeda, K., Ohkura, S. & Tsukamura, H. Physiology of reproduction. in The Laboratory Rat (ed. Krinke, G.) 145–177 (Academic Press, London, UK, 2000).
Marcondes, F.K., Bianchi, F.J. & Tanno, A.P. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 62, 609–614 (2002).
Paccola, C., Resende, C., Stumpp, T., Miraglia, S. & Cipriano, I. The rat estrous cycle revisited: a quantitative and qualitative analysis. Anim. Reprod. 10, 677–683 (2013).
Hashimoto, I. et al. Preovulatory secretion of progesterone, luteinizing hormone, and prolactin in 4-day and 5-day cycling rats. Biol. Reprod. 36, 599–605 (1987).
Gay, V.L., Midgley, A.R. Jr & Niswender, G.D. Pattem of gonadotrophin secretion associated with ovulation. Fed. Proc. 29, 1880–1887 (1970).
Amaral, S., Oliveira, P. & Santos, J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 4, 46–54 (2008).
Wise, P. et al. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog. Horm. Res. 57, 235–256 (2002).
Acknowledgements
We acknowledge the University of Cadiz Central Service of Animal Production and Experimentation (SEPA) for the care and maintenance of our animals and helpful suggestions in this report. We especially recognize the help and assessment of the SEPA Director, Dr. Carlos Costela.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Pinto-Souza, A., Firetto, C., Pérez-Arana, G. et al. Differences in the estrous cycles of Goto-Kakizaki and Wistar rats. Lab Anim 45, 143–148 (2016). https://doi.org/10.1038/laban.980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/laban.980
This article is cited by
-
The Goto-Kakizaki rat is a spontaneous prototypical rodent model of polycystic ovary syndrome
Nature Communications (2021)