Abstract
Objective:
To determine whether intermittent hypoxia (IH) persisting after 36 weeks postmenstrual age (PMA) can be attenuated using caffeine doses sufficient to maintain caffeine concentrations >20 μg ml−1.
Study Design:
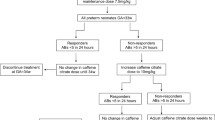
Twenty-seven infants born <32 weeks were started on caffeine citrate at 10 mg kg−1 day−1 when clinical caffeine was discontinued. At 36 weeks PMA, the dose was increased to 14 or 20 mg kg−1 day−1 divided twice a day (BID) to compensate for progressively increasing caffeine metabolism. Caffeine concentrations were measured weekly. The extent of IH derived from continuous pulse oximetry was compared to data from 53 control infants.
Result:
The mean (s.d.) gestational age of enrolled infants was 27.9±2 weeks. Median caffeine levels were >20 μg ml−1 on study caffeine doses. IH was significantly attenuated through 38 weeks PMA compared with the control group.
Conclusion:
Caffeine doses of 14 to 20 mg kg−1 day−1 were sufficient to maintain caffeine concentrations >20 μg ml−1 and reduce IH in preterm infants at 36 to 38 weeks PMA.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ruehland WR, Rochford PD, O'Donoghue FJ, Pierce RJ, Singh P, Thornton AT . The new AASM criteria for scoring hypopneas: impact on the apnea hypopnea index. Sleep 2009; 32 (2): 150–157.
Rhein LM, Dobson NR, Darnall RA, Corwin MJ, Heeren TC, Poets CF et al. Effects of caffeine on intermittent hypoxia in infants born prematurely: a randomized clinical trial. JAMA Pediatr 2014; 168 (3): 250–257.
Charles BG, Townsend SR, Steer PA, Flenady VJ, Gray PH, Shearman A . Caffeine citrate treatment for extremely premature infants with apnea: population pharmacokinetics, absolute bioavailability, and implications for therapeutic drug monitoring. Ther Drug Monit 2008; 30 (6): 709–716.
Mohammed S, Nour I, Shabaan AE, Shouman B, Abdel-Hady H, Nasef N . High versus low-dose caffeine for apnea of prematurity: a randomized controlled trial. Eur J Pediatr 2015; 174 (7): 949–956.
Steer P, Flenady V, Shearman A, Charles B, Gray PH, Henderson-Smart D et al. High dose caffeine citrate for extubation of preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2004; 89 (6): F499–F503.
Steer PA, Flenady VJ, Shearman A, Lee TC, Tudehope DI, Charles BG . Periextubation caffeine in preterm neonates: a randomized dose response trial. J Paediatr Child Health 2003; 39 (7): 511–515.
Brockmann PE, Poets A, Urschitz MS, Sokollik C, Poets CF . Reference values for pulse oximetry recordings in healthy term neonates during their first 5 days of life. Arch Dis Child Fetal Neonatal Ed 2011; 96 (5): F335–F338.
Dobson NR, Liu X, Rhein LM, Darnall RA, Corwin MJ, McEntire BL et al. Salivary caffeine concentrations are comparable to plasma concentrations in preterm infants receiving extended caffeine therapy. Br J Clin Pharmacol 2016; 82 (3): 754–761.
Perera V, Gross AS, McLachlan AJ . Caffeine and paraxanthine HPLC assay for CYP1A2 phenotype assessment using saliva and plasma. Biomed Chromatogr 2010; 24 (10): 1136–1144.
Ross PA, Newth CJ, Khemani RG . Accuracy of pulse oximetry in children. Pediatrics 2014; 133 (1): 22–29.
Ali NJ, Pitson D, Stradling JR . Sleep disordered breathing: effects of adenotonsillectomy on behaviour and psychological functioning. Eur J Pediatr 1996; 155 (1): 56–62.
Bass JL, Corwin M, Gozal D, Moore C, Nishida H, Parker S et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics 2004; 114 (3): 805–816.
Hunt CE, Corwin MJ, Baird T, Tinsley LR, Palmer P, Ramanathan R et al. Cardiorespiratory events detected by home memory monitoring and one-year neurodevelopmental outcome. J Pediatr 2004; 145 (4): 465–471.
Doyle LW, Cheong J, Hunt RW, Lee KJ, Thompson DK, Davis PG et al. Caffeine and brain development in very preterm infants. Ann Neurol 2010; 68 (5): 734–742.
Schmidt B, Anderson PJ, Doyle LW, Dewey D, Grunau RE, Asztalos EV et al. Survival without disability to age 5 years after neonatal caffeine therapy for apnea of prematurity. JAMA 2012; 307 (3): 275–282.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A et al. Long-term effects of caffeine therapy for apnea of prematurity. N Engl J Med 2007; 357 (19): 1893–1902.
Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D et al. Association between intermittent hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 2015; 314 (6): 595–603.
Aranda JV, Collinge JM, Zinman R, Watters G . Maturation of caffeine elimination in infancy. Arch Dis Child 1979; 54 (12): 946–949.
Dobson NR, Hunt CE . Pharmacology review: caffeine use in neonates: indications, pharmacokinetics, clinical effects, outcomes. Neoreviews 2013; 14: e540–e550.
Lee TC, Charles B, Steer P, Flenady V, Shearman A . Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther 1997; 61 (6): 628–640.
McPherson C, Neil JJ, Tjoeng TH, Pineda R, Inder TE . A pilot randomized trial of high-dose caffeine therapy in preterm infants. Pediatr Res 2015; 78 (2): 198–204.
Hunt CE, Corwin MJ, Weese-Mayer DE, Ward SL, Ramanathan R, Lister G et al. Longitudinal assessment of hemoglobin oxygen saturation in preterm and term infants in the first six months of life. J Pediatr 2011; 159 (3): 377–383 e371.
Acknowledgements
This study was supported in part by the American SIDS Institute (ASI), Naples, FL, USA. We thank Denis Rybin, PhD for his active participation in data analysis including statistical analysis; Robert Ward, MD for contributions to study design; Masimo Corporation, for providing the pulse oximeters; and Acumen Instruments Corporation for providing the serial data recorders.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
Dr McEntire is the Chief Executive Officer of the ASI, and Drs Hunt and Dobson are members of the ASI Board of Directors. The sponsor had no role in the analysis and interpretation of data. Dr McEntire participated in design and conduct of the study; collection of data; review of the manuscript; and the decision to submit the manuscript for publication. Drs Dobson, Hunt and Corwin have full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr Dobson wrote the first draft of the manuscript. Dr Rhein reports grants from PCORI and non-financial support from Masimo, outside the submitted work. Dr James is part owner of Acetaminophen Toxicity Diagnostics, LLC, which is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) STTR/2R42DK079387, and she receives salary support for research from the National Center for Advancing Translational Sciences (NCATS) awarded to the Translational Research Institute at the University of Arkansas for Medical Sciences (UL1RR029884). The remaining authors declare no conflict of interest.
Additional information
This study was presented in part at Pediatric Academic Societies Annual Meeting, Baltimore, MD, USA (3 May 2016).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Army, Department of Defense, nor the U.S. Government. Some authors are a military service member or a U.S. Government employee. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. 101 defines a United States Government work as a work prepared by a military service member or employee of the United States Government as part of that person’s official duties.
L Consenstein (Department of Pediatrics, St. Joseph’s Hospital Health Center, Syracuse, NY, USA) and RD White (Pediatrix Medical Group, Memorial Hospital, South Bend, IN, USA).
Rights and permissions
About this article
Cite this article
Dobson, N., Rhein, L., Darnall, R. et al. Caffeine decreases intermittent hypoxia in preterm infants nearing term-equivalent age. J Perinatol 37, 1135–1140 (2017). https://doi.org/10.1038/jp.2017.82
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.82
This article is cited by
-
Plasma serotonergic biomarkers are associated with hypoxemia events in preterm neonates
Pediatric Research (2023)
-
Adverse effects of COVID-19 pandemic on a multicenter randomized controlled trial
Journal of Perinatology (2023)
-
Immature control of breathing and apnea of prematurity: the known and unknown
Journal of Perinatology (2021)
-
Postmenstrual age at discharge in premature infants with and without ventilatory pattern instability
Journal of Perinatology (2020)
-
Caffeine: an evidence-based success story in VLBW pharmacotherapy
Pediatric Research (2018)