Abstract
Objective:
Examine respiratory severity scores (RSS) (mean airway pressure × fraction of inspired oxygen) and resting energy expenditure (REE) on neurally adjusted ventilatory assist (NAVA) compared with synchronized intermittent mandatory ventilation with pressure controlled and supported breath (SIMV (PC)PS).
Study Design:
A randomized, crossover trial in a level IV neonatal intensive care unit. Twenty-four patients were ventilated with NAVA or SIMV (PC) PS for 12 h and then crossed over to the alternative mode for 12 h. The primary outcome (RSS) and additional secondary respiratory outcomes were analyzed.
Results:
RSS and measured REE were not different between modes. On NAVA, peak inspiratory pressures were lower (17.8 vs 19.9 cmH2O (P<0.05)) without higher oxygen requirements. Respiratory rates were higher on NAVA (52 vs 39 (P<0.05)), estimated work of breathing (WOB) (0.01 vs 0.04 J l−1 (P<0.05)) was improved.
Conclusion:
NAVA mode can be safe without increase in RSS or REE. Although respiratory rates were higher, this was offset by lower peak inspiratory pressures and WOB during NAVA.
Similar content being viewed by others
Introduction
Multiple ventilation strategies for infants with respiratory failure exist including noninvasive support to high-frequency oscillation. Advancements in noninvasive ventilation have led to a reduction in mechanical ventilation (MV) in the past 5 years, yet infants still require intubation and MV.1 Currently, there is no consensus regarding the optimal MV strategy for infants with respiratory failure.2
In light of the paucity of head-to-head comparisons of ventilator strategies demonstrating superiority of one mode over the other, ongoing efforts continue to identify ventilation strategies that minimize ventilator-associated lung injury and chronic respiratory morbidity. Neurally adjusted ventilatory assist (NAVA) is a relatively new mode of ventilatory support that delivers respiratory support in response to the electrical activity of the patient’s diaphragm (Edi).3 NAVA uses a gastric tube with eight pairs of electrodes plus a reference electrode mounted within, which systematically detects the neural activity of the diaphragm.4 The degree of respiratory support and synchronization vary proportionately with the signal received. When the Edi falls to 70% of Edi peak, the breath cycles to the expiratory phase. The positive end expiratory pressure continues during this time, yet if the Edi does not return before the operator selected apnea time, then backup ventilation is initiated. NAVA breaths are determined by the intensity and frequency of the electrical stimulus from the diaphragm. The patient determines the initiation of the breath, respiratory rates (RRs), inspiratory times, peak inspiratory pressures (PIPs) and breath termination.4
Previous studies have shown that NAVA is well tolerated in the infant population with improved patient–ventilator synchrony and lower PIPs.5, 6, 7, 8, 9 The primary objective of this study was to measure the respiratory severity score (RSS) (mean airway pressure multiplied by fraction of inspired oxygen) and resting energy expenditure (REE) on NAVA compared with synchronized intermittent mandatory ventilation with pressure controlled and supported breaths (SIMV (PC) PS). In addition, secondary outcomes including ventilator settings, vital signs and pulmonary gas exchange were assessed. To our knowledge, there are no studies evaluating respiratory severity with NAVA and concomitant energy expenditure in infants.
Methods
A single-center, prospective, randomized controlled, crossover comparison of NAVA and SIMV (PC) PS was conducted for a 20-month period from October 2013 through June 2015 in the level IV NICU at Children’s Mercy Hospital in Kansas City, Missouri. Approval for this study was obtained by the Children’s Mercy Hospital Institutional Review Board. The protocol was declared on clinicaltrials.gov (NCT02518230).
Inclusion/exclusion criteria
All infants >22 weeks gestation and requiring MV were screened for inclusion in the study. Subjects were eligible for inclusion if stable on the ventilator (defined as no escalation of ventilator support for 12 h before starting the study) and were expected to remain intubated for >24 h. Patients were excluded if they had phrenic nerve palsy or suppression of respiratory drive due to sedation or neurologic compromise. During the study time period, both modes of ventilation were available and used in routine practice. The ventilation mode was selected by the clinical team caring for the patient and was not guided by specific policy.
Protocol
After screening criteria were met, the principal investigator or a coinvestigator obtained informed consent from the parents or legal guardians. Block randomization in groups of four were used to determine initial treatment assignment of SIMV (PC) PS or NAVA. MV was provided by the Servo-i ventilator (Maquet Critical Care AB, Solna, Sweden). The patient had an Edi catheter (Maquet Critical Care, Solna, Sweden) placed if one was not already present. The catheter position was adjusted using the ECG ‘catheter positioning’ screen to obtain an accurate Edi signal. The initial NAVA level was determined by the principal investigator and/or respiratory therapist by keeping Edi peak values between 5 and 15 mcV, using the NAVA preview mode, to match comparable support provided by SIMV (PC) PS.
Data were collected for 12 h before randomization to determine baseline respiratory characteristics. The infant was randomized to either the SIMV (PC) PS or NAVA mode for 12 h duration. The NAVA level was adjusted to ensure comparable ventilator settings with the SIMV (PC) PS mode. Before data collection, the infants underwent a stabilization period of 1 to 3 h if they were randomized to a mode that was different than what they were on before the start of the study. After stabilization was achieved, data were collected for 12 h. The subject then crossed over to the alternative mode, and after a washout period of 1 to 3 h, data were again collected for 12 h duration. During each mode, indirect calorimetry measurements were obtained to assess estimated energy expenditure while being treated with SIMV (PC) PS or with NAVA.
Data collection
Ventilator settings, measured values and estimated work of breathing data were recorded automatically every minute using the Data Acquisition Macro v.1.3 (Maquet Critical Care AB) on the Servo-I ventilator. Study personnel recorded demographic data, vital signs and blood gas measurements. Respiratory severity score and vital signs including heart rate, RR, blood pressure and pulse oximetry were recorded every 4 h.
Indirect calorimetry
Indirect calorimetry was measured with a portable continuous indirect calorimetry device designed for neonates and infants (Vmax Metabolic Monitor; Viasys Healthcare, Palm Springs, CA, USA). Flow sensor and gas calibration were performed before each measurement. During the measurement, patients were continuously monitored to ensure the oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory quotient remained consistent with <10% variation and steady state of all variables was achieved. If steady state was not reached, the test was extended for 30 min and the average of the results was reported. Oxygen consumption and carbon dioxide production values were measured and the REE was calculated. Each patient underwent one indirect calorimetry measurement during each 12-h period. Timing of indirect calorimetry was not specified during each period. Results were converted from the Vmax output to Excel for statistical analysis.
Sample size
Our previous analysis of 47 infants using NAVA identified a change in RSS from 4.44 (±3.27) before the initiation of NAVA to 3.23 (±1.85) after 12 h of NAVA use.10 Based on the mean and standard deviation from this analysis, our power calculation indicated that a minimal sample size of 22 was needed to detect difference of two in the RSS at an α of 0.05 and a β of 0.80.
Statistical analysis
Normally distributed demographic data was expressed as mean±standard deviation (s.d.) and non-normally distributed data was expressed at medians and interquartile range (IQR). Means±s.d. and medians±IQR were calculated using Microsoft Excel. Paired t-test and Wilcoxon's rank-sum were used to compare parametric and nonparametric variables, respectively. Statistical analysis was performed using SPSS v.20.0 (SPSS Inc., Chicago, IL, USA) and SAS/STAT software v.9.2 (Copyright 2011; SAS Institute Inc., Cary, NC, USA); P<0.05 was considered significant.
Monitoring
During the study, the principal investigator, coinvestigator and statistician reviewed the data and protocol after the first, seventh and seventeenth patient enrolled for evaluation of safety of the study. After each review, the study was deemed safe for continuation.
Results
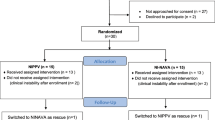
One hundred and forty-nine patients requiring MV in either the SIMV (PC) PS or NAVA mode were screened for potential inclusion in the study. Seventy-nine patients met exclusion criteria and 70 patients were eligible for study inclusion. Twenty-two patients completed the study protocol and were included in the analysis. Two additional patients were enrolled in the study but excluded from the analysis. One patient completed the study but the data collection was incomplete; a second patient developed insufficient spontaneous respiratory drive while on NAVA, so was removed from NAVA mode by the clinical team and was unable to complete the study (Figure 1).
Consort diagram: flow of eligible, consented and enrolled subjects. aOne infant was randomized but data were not collected in entirety, and therefore was not included in analysis. bOne infant was randomized to synchronized intermittent mandatory ventilation with pressure controlled and supported breath (SIMV (PC) PS), but did not tolerate transition to neurally adjusted ventilatory assist (NAVA).
The study population consisted of 13 females and 9 males (Supplementary Information). The median gestational age at birth was 26 4/7 (23 0/7 to 39 0/7) weeks and at the time of study enrollment had a median postmenstrual age of 32 1/7 (28 5/7 to 44 4/7) weeks. The median age at study enrollment was 40 days (3 to 135 days). The median birth weight was 734 g (432 to 3165 g) and at the time of study enrollment had a median weight of 1293 g (883 to 4140 g).
Before study entry, 16 patients were ventilated with SIMV (PC) PS and 6 were ventilated with NAVA. The mean RSS of the patients ventilated with SIMV (PC) PS before the study was 3.31 (±0.87), and the mean RSS of the patients using NAVA before the study was 3.75 (±1.74), P=0.9. There was no difference between the mean airway pressure (MAP) and fraction of inspired oxygen (FiO2) at baseline. The average prestudy MAP with SIMV (PC) PS was 10.3 cmH2O (±1.9), with an average FiO2 of 0.32 (±0.06), and with NAVA, the average MAP was 9.9 cmH2O (±2.3), with an average FiO2 of 0.36 (±0.11), P=0.75 and P=0.5 respectively.
For the outcome of RSS, there was no significant difference between NAVA and SIMV (PC) PS. The change in RSS between the two modes of ventilation for the individual patients is shown in Figure 2. There was no significant difference between NAVA and SIMV (PC) PS in MAP, FiO2 or dynamic compliance. The estimated REE was also similar in both NAVA and SIMV (PC) PS (Table 1). During the NAVA phase of the study, backup ventilation was rare (range of 0 to 2.6% of total NAVA time).
Secondary outcomes showed significant reductions in PIP, tidal volumes and estimated work of breathing (WOB) with an increase in RR on NAVA, although still within normal range for age (Table 1). There was no significant difference in HR, blood pressure or SpO2 (blood oxygen saturation level) for each mode. Blood gas measurements showed a small but significant increase in the carbon dioxide levels while on NAVA.
No acute adverse events occurred for any of the patients during the study. After each infant completed the protocol, the primary medical team determined which mode would be used for the patient. Seventy-five percent of patients remained on the ventilator mode they were on before the start of the study.
Discussion
The results from this study demonstrate that patients receiving support with NAVA have a comparable RSS with similar energy expenditure as compared with ventilation with SIMV (PC) PS at the end of 12 h. NAVA was associated with reduced PIPs, improved estimated WOB, increased RR and higher pCO2 while maintaining an equivalent oxygen requirement and hemodynamic stability. In theory, increased WOB (a calculated measure of the energy needed to achieve the inspiratory pressure above positive end expiratory pressure) or increased energy used while on SIMV (PC) PS suggests unnecessary work by the patients in this mode.11 This could be a potential benefit of NAVA mode where the patient minimizes energy expended to achieve similar support needs. Our results demonstrate that NAVA is as effective as SIMV (PC) PS in short-term use; in this population of infants, this mode of ventilation can be considered when selecting a mode of ventilation for infants.
In a recent non-randomized crossover trial of NAVA and pressure-regulated volume control, Longhini et al.,5 showed that NAVA was as effective as pressure-regulated volume control in maintaining gas exchange while reducing PIPs and improving patient–ventilator interaction and synchrony. Stein et al.6 compared NAVA to pressure controlled ventilation in a non-randomized crossover trial and showed that NAVA was as effective with decreased PIPs, FiO2 and RR with lower PCO2 and improved compliance. Lee et al.7 compared NAVA with SIMV with PS and demonstrated that NAVA lowered PIPs and respiratory work while maintaining an equivalent supply of oxygen and gas exchange. All trials were 12 h or less per mode.
RSS, defined as MAP multiplied by FiO2, is an objective score to estimate the severity of respiratory failure in neonates and infants, especially when arterial blood gas measurements may not be accessible.12 RSS has been used as a substitute for oxygenation index ((MAP × FiO2)/PaO2)) in major randomized control trials and has shown a statistically significant association between the two indices with a high specificity.13 Elevated RSS with prolonged ventilation at day of life 30 has been strongly associated with higher mortality in premature infants and an RSS >6 had the highest risk of respiratory mortality.12 We have shown that NAVA has a comparable RSS to SIMV (PC) PS in short-term use.
Indirect calorimetry provides estimates of energy expenditure based on measurements of carbon dioxide production and oxygen consumption during rest and steady-state exercise. It has been validated in ventilated preterm infants weighing as little as 500 g.14, 15 In critically ill, ventilated children, maintaining optimal nutrition is associated with decreased mortality at 60 days, suggesting an important avenue for improving clinical outcomes.16 In a recent study, examining protein and energy expenditure in critically ill ventilated infants and children, a minimum of 57 kcal kg−1 per day with 1.3 g kg−1 per day of protein was required to achieve nitrogen equilibrium.17 Energy expenditure varies between critically ill children (median 40 to 64 kcal kg−1 per day) while variation within an individual child is small.18 In two small cohorts of neonatal patients, energy expenditure ranged from 32 to 79 kcal kg−1 per day.14, 19 In our study, REE was 26.7 kcal kg−1 per d for infants in SIMV (PC) PS and 25.6 kcal kg−1 per day in NAVA. The reason for a lower REE in this study compared with other studies is unclear. The changes in ventilators over time and the variety of patient ages and disease processes included in our study group make direct comparison with these other patient populations difficult.
A strength of this study is the generalizability to a population of neonatal patients ventilated at a broad postmenstrual age ranges in level IV NICUs. This population is at high risk for pulmonary morbidity; thus, a population of infants where efforts to improve outcomes attributable to strategies of assisted ventilation therapy is needed. Although this could also be considered a weakness, the crossover design has inherent strengths, which account for this heterogeneity as the patient act as their own control. Therefore, fewer numbers of patients are needed to achieve the same level of statistical power.
The 12-h duration in each mode of the crossover may be a limitation of the study, yet it is one of the longest NAVA crossover study to date in infants. This study only provides short-term outcome data in patients on stable ventilator support. It may not generalizable to infants with acute or severe respiratory failure in which requirements appear to be rapidly changing. Indirect calorimetry completed during each arm of the crossover may not be reflective of caloric consumption with prolonged ventilator use.
In conclusion, we found that NAVA does not improve clinically important outcomes (MAP, FiO2, RSS and REE) when compared with SIMV (PC) PS. Further studies with longer duration and larger populations of preterm infants would be beneficial to better understand the optimal use and potential impact on decreasing respiratory morbidities for both modes of ventilation.
References
Vendettuoli V, Bellù R, Zanini R, Mosca F, Gagliardi L . Italian Neonatal Network. Changes in ventilator strategies and outcomes in preterm infants. Arch Dis Child Fetal Neonatal Ed 2014; 99: 321–324.
Roumiantsev S . Invasive mechanical ventilation in premature infants: where do we stand today? J Pulmon Resp Med 2013; S13: 1–8.
Sinderby C, Beck J . Neurally adjusted ventilatory assist (NAVA): an update and summary of experiences. Neth J Crit Care 2007; 11: 243–252.
Stein H, Firestone K . Application of neurally adjusted ventilatory assist in neonates. Semin Fetal Neonatal Med. 2014; 19: 60–69.
Longhini F, Ferrero F, De Luca D, Cosi G, Alemani M, Colombo D et al. Neurally adjusted ventilatory assist in preterm infants with acute respiratory failure. Neonatology 2015; 107: 60–67.
Stein H, Alosh H, Ethington P, White DB . Prospective crossover comparison between NAVA and pressure control ventilation in premature neonates less than 1500 grams. J Perinatol 2013; 33: 452.
Lee J, Kim HS, Sohn JA, Lee JA, Choi CW, Kim EK et al. Randomized crossover study of neurally adjusted ventilatory assist in preterm infants. J Pediatr 2012; 161: 808–813.
Breatnach C, Conlon NP, Stack M, Healy M, O'Hare BP . A prospective crossover comparison of neurally adjusted ventilatory assist and pressure-support ventilation in a pediatric and neonatal intensive care unit population. Pediatr Crit Care Med 2010; 11: 7–11.
Kallio M, Koskela U, Peltoniemi O, Kontiokari T, Pokka T, Suo-Palosaari M et al. Neurally adjusted ventilator assist (NAVA) in preterm newborn infants with respiratory distress syndrome—a randomized controlled trial. Eur J Pediatr 2016; 175: 1175–1183.
Rosterman JL, Pallotto EK, Truog WE, Manimtim WM . Neurally adjusted ventilatory assist (NAVA) in neonates and infants: does it work? Poster Session Presented at the American Academy of Pediatrics (AAP) 2012 New Orleans, LA,19–21.
Maquet SERVO Education Study Guide, version 1.14, p 296.
Iyer NP, Mhanna MJ . Non-invasively derived respiratory severity score and oxygenation index in ventilated newborn infants. Pediatr Pulmonol 2013; 48: 364–369.
Malkar MB, Gardner WP, Mandy GT, Stender MR, Nelin LD, Shepherd EG et al. Respiratory severity score on day of life 30 is predictive of mortality and the length of mechanical ventilation in premature infants with protracted ventilation. Pediatr Pulmonol 2015; 50: 363–369.
Shortland GJ, Fleming PJ, Walter JH . Validation of a portable indirect calorimetry system for measurement of energy expenditure in sick preterm infants. Arch Dis Childhood 1992; 67: 1207–1211.
Thureen PJ, Phillips RE, DeMarie MP, Hoffenberg A, Bronstein MN, Spedale SB et al. Technical and methodologic considerations for performance of indirect calorimetry in ventilated and nonventilated preterm infants. Crit Care Med 1997; 25: 171–180.
Mehta NM, Bechard LJ, Cahill N, Wang M, Day A, Duggan CP et al. Nutritional practices and their relationship to clinical outcomes in critically ill children—an international multicenter cohort study. Crit Care Med 2012; 40: 2204–2211.
Jotterand Chaparro C, Depeyre JL, Longchamp D, Perez MH, Taffe P, Clotting J . How much protein and energy are needed to equilibrate nitrogen and energy balances in ventilated critically ill children? Clin Nutr 2015; 35 (2): 460–467.
de Klerk G, Hop WC, de Hoog M, Joosten KF . Serial measurements of energy expenditure in critically ill children: useful in optimizing nutritional therapy? Intens Care Med 2002; 28: 1781–1785.
Jaksic T, Shew SB, Keshen TH, Dzakovic A, Jahoor F . Do critically ill surgical neonates have increased energy expenditure? J Pediatr Surg 2001; 36: 63–67.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors have contributed to this manuscript and declare no conflict of interest. This project was funded in part through the Graduate Medical Education Fellowship program (Jamie Rosterman, DO) and the Center for Infant Pulmonary Disorders located at Children’s Mercy-Kansas City.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
About this article
Cite this article
Rosterman, J., Pallotto, E., Truog, W. et al. The impact of neurally adjusted ventilatory assist mode on respiratory severity score and energy expenditure in infants: a randomized crossover trial. J Perinatol 38, 59–63 (2018). https://doi.org/10.1038/jp.2017.154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.154