Abstract
Objective:
The purpose of this study was to test a specialized needs-based management model for a high volume of babies born with neonatal abstinence syndrome (NAS) while controlling costs and reducing neonatal intensive care unit (NICU) bed usage.
Study Design:
Data were analyzed from inborn neonates >35 weeks’ gestational age with the diagnosis of NAS (ICD9-CM 779.5), requiring pharmacologic treatment and discharged from 2010 through 2015. Significance was determined using Kruskal–Wallis and Mann–Whitney as well as χ2 for trend.
Results:
NAS requiring medication treatment increased from 34.1 per 1000 live births in 2010 to 94.3 per 1000 live births in 2015 (P<0.0001 for trend). Hospital charges were significantly different in the three described locations (P<0.0001). Median per patient hospital charges for medically treated NAS were $90 601 (interquartile range (IQR) $64 489 to $128 135) for NAS patients managed in the NICU, $68 750 (IQR $44 952 to $92 548) for those managed in an in-hospital dedicated unit and $17 688 (IQR $9933 to $20 033) for those cared for in an outpatient neonatal withdrawal center. NICU admission was avoided in 78% of the population once both alternative locations were fully implemented.
Conclusions:
In this cohort of infants, a 219% increase in the number of infants treated for NAS overwhelmed the capacity of our traditional resources. There was a need to develop new treatment approaches dealing with the NAS crisis and a growing population of prenatally exposed babies. We found that the described model of care significantly reduced charges and stabilized admissions to our NICU despite the marked increase in cases. Without this system, our NICU would be in a critical state of gridlock and diversion; instead, we have efficient management of a large NAS population.
Similar content being viewed by others
Introduction
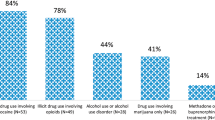
Although substance abuse is a concern nationwide,1, 2 the state of West Virginia faces distinct challenges due to issues related to rurality, poverty, and incidence of licit and illicit opioid use. In Cabell County, WV, USA, we have seen sharp increases in the rates of opioid overdoses (from 146 in 2012 to 944 in 2015) and drug overdose deaths (from 39 to 92 over the same years) despite a decrease in the overall population (Lemley, unpublished). This increased prevalence of drug abuse has created a comparable increase in prenatally exposed babies experiencing neonatal abstinence syndrome (NAS).
NAS is a postnatal drug withdrawal syndrome that primarily occurs after in utero exposure to opioids.3 In the United States, it is estimated that one infant is born every 25 min with NAS representing $1.5 billion in additional hospital charges.4, 5 NAS is increasing in frequency and can represent a large percentage of admissions to some NICUs.6 Among 28 states with publicly available data in the Healthcare Cost and Utilization Project during 1999 to 2013, the overall NAS incidence increased 300% from 1.5 per 1000 hospital births in 1999, to 6 per 1000 hospital births in 2013. Using state-based data, the CDC reports that WV has the highest rate of babies born with NAS in 2013 at 33.4 per 1000 births.7 These data may be an underestimate as neonatal transfers were excluded. It has also been reported using the WV Health Care Authority database and the Uniform Billing database that the Southeastern region of WV has the highest incidence of NAS in the state at 48.76 per 1000 births.8 The goals of this report were to describe the marked increase in prevalence of infants managed for NAS at Marshall University’s major teaching institution, Cabell Huntington Hospital, and the resulting impact on health-care utilization and costs for our NICU. We will also share a model for dealing with the pressures of this epidemic by creating dedicated inpatient and stand-alone facilities to care for these patients.
Methods
Participants
We searched the hospital database at our institution for all inborn neonates >35 weeks’ gestational age with the diagnosis of NAS (International Classification of Diseases, 9th Edition, Clinical Modification (ICD-10-CM) 779.5) requiring pharmacotherapy and discharged from 1 January 2010 through 31 December 2015. NICU patients who required opioids for their medical management and subsequently developed iatrogenic NAS were excluded. Neonatal transfers from outside facilities who required treatment for NAS were also excluded as they would misrepresent our analysis of affected infants as a percentage of inpatient births. Data were collected and reviewed after approval of the Marshall University Institutional Review Board.
Procedures
Cabell Huntington Hospital served as a tertiary care perinatal referral center for Southern West Virginia, Eastern Kentucky and Southeastern Ohio, and thus this report included drug exposed maternal patients referred for delivery from throughout the tristate area. Neonates born at our institution were initially admitted to the newborn nursery unless medical issues such as respiratory distress, infection or metabolic problems necessitated NICU care. All mothers on admission for delivery at this hospital had a urine drug screen. The policy of mandatory maternal drug testing was enacted in mid-2012 so that all mothers delivering at our institution received a urine drug screen. Any mother with a positive drug screen at delivery, a history of positive screen during pregnancy, past history of drug abuse or admission of drug use during pregnancy was followed up with an umbilical cord tissue toxicology at delivery (United States Drug Testing Laboratories, Des Plaines, IL, USA). All newborns were observed for signs of NAS using the Finnegan Scoring Tool.9 If symptoms of NAS developed, the neonate was managed using a protocol for treatment of NAS previously described.10 Because of concerns regarding the local population, outpatient administration of opioids was avoided.
System of care
Prior to August 2012, neonates treated for withdrawal were managed in the NICU, a 36-bed, level III unit with an average of 550 admissions per year. Following the American Academy of Pediatrics recognition that the environment and approach to neonates with prenatal drug exposure differed from that of other NICU patients,11 our institution opened the neonatal therapeutic unit (NTU) in July of 2012. The unit existed for the purposes of providing a safe environment in the hospital to care for neonates with NAS as well as opening beds in the NICU to care for the medical and surgical problems for which it was designed. The NTU was a 15-bed low-light, low-noise unit with a dedicated nursing staff and nursing aids along with community volunteers called ‘rockers’ who were specially trained to hold and rock the neonates when appropriate and when family members were unavailable. The staff utilized therapeutic handling techniques that further enhanced calming effects on the neonates. Hospital administration was supportive of the establishment of the NTU due to the high rate of diversion away from the NICU the previous 6 months; consequently, five vacated labor and delivery rooms were converted to accommodate the new unit. The isolated area was important for success. Because of space constraints, rooming-in was unavailable for parents; however, parental involvement was encouraged. Two to three infants shared individual rooms. Because newborn beds were not considered acute care beds, the addition of the NTU did not alter the total hospital bed count or require State authorization. Medical care was provided by a core group of pediatricians. After the NTU was established, all babies with NAS requiring medication treatment were transferred from the newborn nursery to the NTU. If NTU beds were unavailable, the neonate was transferred to the NICU for treatment. The NICU served primarily as an overflow for patients from the NTU and occasionally for babies with other medical problems.
In October of 2014, an offsite facility called Lily’s Place was opened to serve as a neonatal abstinence center with 12 beds and a dedicated staff of nurses, patient care assistants and volunteers trained in the care of neonates with NAS. In addition, on-site social service personnel served to transition caretakers (birth parents, foster parents or adopting families) from Lily’s Place to a safe home environment. Limited number of patient rooms were available to accommodate rooming-in, thus that space was utilized to help prepare caretakers prior to discharge.
Lily’s Place was a nonprofit 501c3 facility that provided a safe recovery environment for the infant, and offered parental education and made referrals to addiction-recovery programs for caregivers when appropriate. The 7500 square foot facility, previously a physician’s office building, was donated and renovated by community volunteers and grant funded staff to serve as an outpatient neonatal abstinence center. Revenue from operations, grants and gifts last year totaled $1 300 000 ($850 000 from operations and $450 000 from grants and gifts) and expenses totaled $1 300 000. Medical care was provided by a single neonatologist. A step-by-step description of replicating the creation of a neonatal withdrawal center was recently published12 and an updated replication plan is available at http://www.lilysplace.org.
After creation of Lily’s Place, all inpatient newborns were admitted at birth to newborn nursery or NICU if comorbidities existed. When it was determined that medication was required for treatment of NAS, infants were moved to NTU or secondarily to NICU when beds were unavailable. After initial assessment and stabilization, neonates could be sent to Lily’s Place when beds were available. Babies were preferentially transferred to Lily’s Place who were considered to potentially benefit from private rooms with less external stimulation. The protocol for medication management of NAS was the same for the NICU, NTU and Lily’s Place.
Statistics
For the purpose of this study, length-of-stay (LOS) and financial data were only applied to neonates during the period of time while they received medication treatment for withdrawal and the 72 h of observation following discontinuation of the medication. For instance, days and charges in the newborn nursery prior to the onset of withdrawal symptoms were not included in this report. Only overflow infants managed in the NICU without comorbidities or those cared for in the NICU prior to the opening of NTU were included in the financial and LOS analyses. The financial and LOS information reported for those infants treated at Lily’s Place included days of medication treatment in NICU or NTU prior to transfer. Patients at Lily’s Place often had a 1 to 3 days stay in the NTU to establish treatment response prior to transfer. This additional stay for the cohort in Lily’s Place was included in the median LOS and charge calculations. Custody issues can cause extended LOS in the NTU and Lily’s Place, and were not accounted for in the calculation.
Neonates were grouped into 1-year periods based on year of discharge for all trend analyses. χ2-trend analysis was used to determine whether there was a significant positive or negative yearly trend. Significance for hospital charges per patient was determined using Kruskal–Wallis test. Mann–Whitney was used to test significance between charges for individual units (NICU vs NTU, NTU vs Lily’s Place and NICU vs Lily’s Place).
Ancillary charge data were converted to hospital cost estimates by multiplying total charges with the appropriate cost-to-charge ratio for NAS at our institution. The cost-to-charge ratio used for this study was 29%. Lily’s Place uses a unique ‘all-in’ charge model designed to be sustainable with a primarily Medicaid population, thus the cost-to-charge ratio is 100%.
Results
Over the entire length of the study, 11.23% of the live births delivered at our institution were verified as prenatally exposed to drugs by cord tissue sample or maternal urine screen with 18.26% over the last 3 years. Our study population consisted of 1023 of the prenatally exposed infants who required pharmacotherapy. NAS births requiring pharmacotherapy per 1000 live births increased from 34.1 in 2010 to 94.3 in 2015 (P<0.0001 for trend) (Table 1). In the same time period, the number of babies known to be prenatally exposed to neuroactive substance per 1000 increased from 29.1 to 185.8 (P<0.0001 for trend) (Figure 1).
NAS incidence per 1000 live births at Cabell Huntington Hospital (CHH). Neonates with proven prenatal exposure to neuroactive substances and neonates pharmacologically treated with methadone diagnosed with NAS per 1000 live births per year. Trend is significant as determined by χ2-test for trend (P<0.0001). West Virginia (WV) NAS diagnoses are as reported in the literature for comparison.1 WV counts of NAS not available for 2014 to 2015. NAS, neonatal abstinence syndrome.
The percentage of total NAS patients requiring pharmacologic treatment in the NICU fell from 100% prior to the establishment of the NTU to 22% by 2015 (Figure 2). For each year from 2011 through 2015, the yearly percentage of total NICU admission days for NAS was 5.2%, 11.3%, 16.2%, 13.6% and 15.4%, respectively. The yearly percentage has stabilized despite a large increase in the total number of NAS patients seen at our institution.
In a comparison of NICU admissions from the 6 months prior to the opening of the NTU to 6 months after the opening of the NTU, the average daily census in the NICU fell from 31.6 babies to 26.6 babies. In the same time period, the time on diversion and unable to accept transports decreased from 110 days to 12 days, and the number of referred infants or high-risk mothers diverted to other tertiary care facilities dropped from 55 to 6. From its opening in October of 2014 through December 2015, Lily’s Place cared for 78 prenatally exposed and treated neonates.
The median LOS for NAS medication-treated patients in the NICU without comorbidities was 24 days (interquartile range 24 to 52). Median LOS in the NTU was 26 days (interquartile range 26 to 42). The median LOS for Lily’s Place was 33 days (interquartile range 32 to 60), indicative of the transition to home life mission of the facility in addition to extended neonatal withdrawal care. The average daily charge distribution was significantly different between the NICU and NTU (P<0.0001), the NTU and Lily’s Place (P<0.0001) and the NICU and Lily’s Place (P<0.0001) (Figure 3). Median charges per patient were $90 601 in the NICU, $68 750 in the NTU and $17 688 at Lily’s Place (Table 2).
Charge distribution for NAS patients in different units. Median charge per patient (5 to 95%). Significance for hospital charges per patient was determined using Kruskal–Wallis test (P<0.0001). Mann–Whitney was used to test significance between charges for individual units (NICU vs NTU: P<0.0001), (NTU vs Lily’s Place: P<0.0001) and (NICU vs Lily’s Place: P<0.0001). NAS, neonatal abstinence syndrome; NICU, neonatal intensive care unit; NTU, neonatal therapeutic unit.
Discussion
From 2004 through 2013, in 299 nationwide centers, the total percentage of NICU days attributed to NAS increased from 0.6 to 4.0% with eight centers reporting >20% of all NICU days attributed to NAS.6 The number of pharmacologically treated NAS neonates at our institution rose from 83 in 2010 to 265 in 2015. Despite a 219% increase in pharmacologically treated NAS (equating to about 364 additional hospital days), we have been able to maintain NAS admissions in the NICU to <17%. As we confronted the increase in NAS patients and, in response, developed this system, it became clear that we had underestimated the severity in our own population. The NTU and Lily’s Place proved essential as our 36-bed NICU would not have been able to effectively operate with that volume.
Nationally, mean hospital charges for discharges with NAS increased from $39 400 in 2000 to $53 400 in 2009. By 2009, 77.6% of charges for NAS were attributed to state Medicaid programs.4 The estimated hospital charge in 2012 for pharmacologically treated NAS was reported to be $93 400 (86 900 to 100 000).5 In 2015, the median charges were very similar for NAS treated in the NICU at our institution, but we have also developed reduced charge options for NAS patients with the NTU and Lily’s Place.
Our study contains limitations that merit discussion. Although we report a cost-to-charge ratio conversion, it is important to note that this is only an estimate of actual direct cost that our current accounting system does not allow us to determine. This study has been undertaken at a single institution and the findings might not be applicable to other centers. However, our larger population at a single site makes longitudinal studies more promising. We only report those patients with proven prenatal exposure and diagnosed NAS that required pharmacologic treatment. Our institution is a regional referral center for perinatal care. It is possible that mothers with prenatal drug use were referred or selected to deliver at our institution from outside the immediate region making it difficult to determine the true incidence of NAS for a discrete population area.
It has been suggested that active maternal participation is the best nonpharmacological care for NAS.11 Several studies have reported rooming-in with active parental involvement has reduced LOS and lowered cost for NAS.13, 14, 15 Saiki et al.16 showed some improvements in duration of treatment and LOS using a postnatal ward in a small population (n=18), but they also included increased parental involvement. While we have remained committed to parental involvement in treating NAS in all our units, limited space availability has made achieving this goal problematic in this epidemic environment. Previous studies have demonstrated a reduced LOS and decreased hospital charges by transitioning pharmacologic treatment from an inpatient to an outpatient setting.17, 18 Others, however, have abandoned outpatient management because of concerns raised by community pediatricians regarding NAS management.19
Furthermore, we cannot present objective data to explain the longer duration of hospitalization for treatment of NAS at Lily’s Place when compared to NICU and NTU. We hypothesize that neonates who were more difficult to capture on therapy or slower to wean were selectively transferred to Lily’s Place to benefit from private rooms with less external stimulation.
In this report, we have demonstrated a 219% increase in the number of infants treated for NAS over a 6-year study period at our institution overwhelming the capacity of our traditional resources. We have described a model involving the creation of a dedicated inpatient unit and a stand-alone outpatient facility to manage this growing population while stabilizing NICU admissions and reducing charges. We speculate that this system, or a similar system, may become an essential part of neonatal care in many areas due to the growing substance abuse epidemic.
References
Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, Grider JS et al. Opioid epidemic in the United States. Pain Physician 2012; 15 (3 Suppl): ES9–38.
Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW . Rural and urban differences in neonatal abstinence syndrome and maternal opioid use, 2004 to 2013. JAMA Pediatr 2016; 171 (2): 194–196.
Kocherlakota P . Neonatal abstinence syndrome. Pediatrics 2014; 134 (2): e547–e561.
Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM . Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA 2012; 307 (18): 1934–1940.
Patrick SW, Davis MM, Lehmann CU, Cooper WO . Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol 2015; 35 (8): 650–655.
Tolia VN, Patrick SW, Bennett MM, Murthy K, Sousa J, Smith PB et al. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N Engl J Med 2015; 372 (22): 2118–2126.
Ko JY, Patrick SW, Tong VT, Patel R, Lind JN, Barfield WD . Incidence of neonatal abstinence syndrome - 28 states, 1999-2013. MMWR Morb Mortal Wkly Rep 2016; 65 (31): 799–802.
Stabler ME, Long DL, Chertok IR, Giacobbi PR Jr, Pilkerton C, Lander LR . Neonatal abstinence syndrome in West Virginia substate regions, 2007-2013. J Rural Health 2016; 33 (1): 92–101.
Finnegan LP, Connaughton JF Jr, Kron RE, Emich JP . Neonatal abstinence syndrome: assessment and management. Addict Dis 1975; 2 (1–2): 141–158.
Loudin S, Murray S, Prunty L, Davies T, Evans J, Werthammer J . An atypical withdrawal syndrome in neonates prenatally exposed to gabapentin and opioids. J Pediatr 2017; 181: 286–288.
Hudak ML, Tan RC Committee On Drugs; Committee On Fetus and Newborn; American Academy of Pediatrics. Neonatal drug withdrawal. Pediatrics 2012; 129 (2): e540–e560.
Brown MC, Murray S, Edmunds R, Loudin S . How to Create a Neonatal Withdrawal Center: a New Model of Care for Neonatal Abstinence Syndrome. CreateSpace Independent Publishing Platform: North Charleston, SC, USA, 2015.
Holmes AV, Atwood EC, Whalen B, Beliveau J, Jarvis JD, Matulis JC et al. Rooming-in to treat neonatal abstinence syndrome: improved family-centered care at lower cost. Pediatrics 2016; 137 (6): e20152929.
Hunseler C, Bruckle M, Roth B, Kribs A . Neonatal opiate withdrawal and rooming-in: a retrospective analysis of a single center experience. Klin Padiatr 2013; 225 (5): 247–251.
Hodgson ZG, Abrahams RR . A rooming-in program to mitigate the need to treat for opiate withdrawal in the newborn. J Obstet Gynaecol Can 2012; 34 (5): 475–481.
Saiki T, Lee S, Hannam S, Greenough A . Neonatal abstinence syndrome—postnatal ward versus neonatal unit management. Eur J Pediatr 2010; 169 (1): 95–98.
Backes CH, Backes CR, Gardner D, Nankervis CA, Giannone PJ, Cordero L . Neonatal abstinence syndrome: transitioning methadone-treated infants from an inpatient to an outpatient setting. J Perinatol 2012; 32 (6): 425–430.
Lee J, Hulman S, Musci M Jr, Stang E . Neonatal abstinence syndrome: influence of a combined inpatient/outpatient methadone treatment regimen on the average length of stay of a Medicaid NICU population. Popul Health Manag 2015; 18 (5): 392–397.
Napolitano A, Theophilopoulos D, Seng SK, Calhoun DA . Pharmacologic management of neonatal abstinence syndrome in a community hospital. Clin Obstet Gynecol 2013; 56 (1): 193–201.
Acknowledgements
This report was made possible, in part, with support from the Healthy Connections Coalition and the Appalachian Clinical and Translational Science Institute at Marshall University. We thank Todd Gress, MD, for review of an earlier version of this article and assistance with statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Loudin, S., Werthammer, J., Prunty, L. et al. A management strategy that reduces NICU admissions and decreases charges from the front line of the neonatal abstinence syndrome epidemic. J Perinatol 37, 1108–1111 (2017). https://doi.org/10.1038/jp.2017.101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2017.101
This article is cited by
-
Inclusion of Positive Self-reporting by Mothers of Substance Exposed Neonates Increases the Predictability of NAS Severity Over Toxicology Alone
Maternal and Child Health Journal (2020)