Abstract
About 99% of neonatal deaths occur in low- and middle-income countries. There is a paucity of information on the exact timing of neonatal deaths in these settings. The objective of this review was to determine the timing of overall and cause-specific neonatal deaths in developing country settings. We searched MEDLINE via PubMed, Cochrane CENTRAL, WHOLIS and CABI using sensitive search strategies. Searches were limited to studies involving humans published in the last 10 years. A total of 22 studies were included in the review. Pooled results indicate that about 62% of the total neonatal deaths occurred during the first 3 days of life; the first day alone accounted for two-thirds. Almost all asphyxia-related and the majority of prematurity- and malformation-related deaths occurred in the first week of life (98%, 83% and 78%, respectively). Only one-half of sepsis-related deaths occurred in the first week while one-quarter occurred in each of the second and third to fourth weeks of life. The distribution of both overall and cause-specific mortality did not differ greatly between Asia and Africa. The first 3 days after birth account for about 30% of under-five child deaths. The first week of life accounts for most of asphyxia-, prematurity- and malformation-related mortality and one-half of sepsis-related deaths.
Similar content being viewed by others
Introduction
Of the 6.3 million under-five child deaths that occur globally every year, about 44% occur in the neonatal period.1, 2 Millennium Development Goal 4, which stipulates a reduction of two-thirds in the under-five mortality rate by 2015, obviously cannot be achieved without a substantial reduction in the neonatal mortality rate (NMR).
About 99% of neonatal deaths occur in low- and middle-income countries.3 Unlike the remaining 1% of deaths from resource-replete nations, deaths in these countries do not attract sufficient attention from researchers, policy-makers and other key stakeholders. Indeed, many of them happen at home and are often unrecorded.3 Consequently, there is a paucity of information on why and when these neonatal deaths occur.
A number of reviews have reported the causes of neonatal deaths in developed and developing countries in the last few years.1, 4 However, the timing of neonatal deaths, particularly the distribution of cause-specific deaths, has not been evaluated in detail. Knowledge of the timing of neonatal deaths may help policy-makers to devise optimal delivery strategies of interventions that are proven to be effective in reducing the NMR. For example, if an intervention such as postnatal contacts between mother–infant dyads and health-care providers is known to reduce sepsis-related neonatal mortality rates, prior knowledge of the timing of sepsis-related deaths would help determine the optimal frequency and timing of delivery of that intervention.
Only a few studies have reported the timing of neonatal deaths in developing country settings. No efforts appear to have been made to synthesize the data from these studies in a systematic manner. In the Neonatal Survival Series paper published in The Lancet in 2005, Lawn et al.3 reported the daily risk of death in the first month of life based on the analysis of 47 data sets from the Demographic and Health Surveys of different countries pertaining to the period between 1995 and 2003. Given the need for recent information in a simpler format (that is, proportions rather than ‘risk of death per 1000 survivors’ at different time points), the inherent limitations of data from the household surveys, and the lack of information on the timing of cause-specific neonatal deaths, we conducted this systematic review on the distribution of overall and cause-specific deaths in the first 28 days of life.
Methods
Types of studies/participants
All community-based studies from developing countries that reported the timing of overall or cause-specific neonatal deaths were eligible for inclusion in this review. We used the list by the United States Agency for International Development in 2012 for the identification of developing countries.5
Outcome measures
The outcome variable was the distribution of overall and cause-specific deaths in the first 4 weeks of life. For cause-specific deaths, we looked at the distribution of the four major causes: perinatal asphyxia; sepsis; prematurity; and congenital malformations. For the purpose of the review, we accepted the definitions used by the authors of the individual studies to define the individual causes of death. The single most important cause of death, as defined/classified by the study authors, was used if multiple causes were assigned. We included deaths due to septicemia, pneumonia, tetanus and diarrhea under sepsis-related deaths.
Search methodology
Two review authors (CKN and MJS) independently searched Medline (1966 to June 2012) via PubMed, Cochrane CENTRAL (The Cochrane Library, Issue 6, June 2012) and WHOLIS (1986 to June 2012). We used two strategies to search these databases: (‘Infant, Newborn’[MeSH]) AND (death OR mortality) AND (causes OR timing); (neonatal mortality) AND (causes) AND (distribution). Searches were limited to human studies published in the last 10 years. In addition, we reviewed the reference lists of studies identified by the above methods. We updated the search in December 2013. We also searched CABI Global Health database (December 2013).
We scanned the title and abstract of the retrieved citations to exclude those that were obviously irrelevant. We obtained the full text of the remaining studies to identify the relevant articles.
Data extraction
Data extraction was carried out using a form designed and pilot tested by the authors. Three authors (CKN, RRD and MJS) independently extracted the data from included studies, including year, setting (country, type of population, socioeconomic status, baseline neonatal mortality, gestation and birth weight of infants) and results (number of deaths, timing, causes and so on). Disagreements in extracted data were resolved through discussion with the fourth author (RA).
Data abstraction and synthesis
The following list depicts the steps used to summarize the data from the included studies.
-
1
We first made a master chart with the day of life in columns and different studies in rows. For the first week of life, we made columns for each day—that is, day 0, day 1 and so on. For the next 3 weeks, we made columns for the week(s) rather than days (week 2 and weeks 3 to 4), as it was unlikely that the studies would have provided daily mortality rates for the second week onwards.
-
2
As it was likely that different studies would have used different terminologies to describe the day of birth, we assumed the following to ensure uniformity across the studies:
-
We considered 0 to 23 h as day 0 of life, 24 to 47 h as day 1, 48 to 71 h as day 2 and so on for studies that mentioned the age in hours.
-
For studies that did not mention the age in hours but reported it as <1 day, day 1, day 2 and so on, <1day was considered as ‘day 0’.
-
For those studies that did not report either of these two, we examined whether they had reported days 1 to 28 or days 0 to 27 as the neonatal period. For studies reporting deaths from days 1 to 28, we considered day 1 of the study to be ‘day 0’ for our analysis. Similarly, day 2 was considered as ‘day 1’, day 3 as ‘day 2’ and so on. For studies that reported deaths from day 0 to day 27, we used the data as such—that is, day 0 of the study is ‘day 0’. For those studies that provided data for 0 to 28 days, we included day 7 figures in the data for the second week.
-
-
3
We then attempted to pool the data for different time points. For pooling, we included only those studies that provided data for at least three time points in the first 4 weeks of life (for example, day 1, day 2 and days 3 to 28; days 1 to 3, days 4 to 7, days 8 to 28; and so on). Studies that provided data for only two time points were simply enumerated in the table but not included in the final analysis.
-
4
Pooling of the data was done in three steps:
-
Initially, the data from those studies that provided data for all the time points were pooled to obtain the summary estimate (proportion) for each time point.
-
We then used this summary estimate to calculate the proportion for the missing time points in the other studies (extrapolation). For example, if a study provided data for day 1, day 2 and days 3 to 28, we split the data of 3 to 28 days into data for day 3, day 4, day 5, day 6, week 2 and weeks 3 to 4 based on the proportion obtained from step (i) for these time points.
-
The data—original and extrapolated—were pooled again to obtain the final estimate for each time point.
-
-
5
We made the final graph based on the pooled result for each day in the first week and also for week 1, week 2 and weeks 3 to 4.
-
6
For cause-specific mortality, the same steps were carried out for each of the major outcomes—asphyxia, prematurity, sepsis/pneumonia/diarrhea, congenital malformations and others. The denominator was the total number of deaths secondary to a particular cause (for example, asphyxia) while the numerator was the number of deaths due to that cause on a given day.
Statistical analysis
Data from the studies were entered into Microsoft Excel 2007 (Microsoft Corporation, Redmond, CA, USA). We used the user-written ‘metan’ command in Stata 11.1 (StataCorp, College Station, TX, USA) to pool the results of different studies. We specified the weighting to be used in the meta-analysis by using the ‘wgt()’ option. The total number of enrolled neonates in a given study was used for assigning weight to that study. We applied continuity correction for zero event studies using a correction factor of 0.1. Subgroup analysis based on World Health Organization (WHO) regions was carried out by disaggregating results with the user-written ‘metan’ command (by option) in Stata.6 The final pooled data for each time point were entered into an Excel spreadsheet to generate graphs.
Results
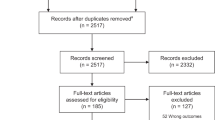
We retrieved 13 306 citations of which 12 805 articles were excluded after screening the title and abstracts, and removing the duplicates. Of the remaining 137 citations, we screened 132 full-text articles. After exclusion of studies from developed countries, as well as studies that did not report outcomes of interest, 21 were found to be eligible for inclusion in the review (Figure 1). Of them, 15 were prospective,7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 4 were retrospective22, 23, 24, 25 and two were cross-sectional studies.26, 27
Overall neonatal mortality
A total of 16 studies reported day-specific mortality (Table 1).7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 22, 23, 24, 26, 27 Data from 14 studies were used to obtain the estimated proportions at the prespecified time points.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 22, 23, 26, 27 Of these, 7 studies provided data for all time points,7, 8, 9, 10, 11, 22, 26 whereas another 7 provided data only for some.12, 13, 14, 15, 16, 23, 27 We extrapolated the pooled results of the former seven studies to calculate the proportion of the missing time points in the latter studies (Supplementary Appendix 1). Two other studies also provided the distribution of neonatal mortality, but their data could not be included in the final analysis (Table 1).17, 24 Most studies used a verbal autopsy (VA) method to determine the cause of death.
Figure 2 depicts the distribution of overall neonatal deaths in the first 4 weeks of life. Of the total deaths that occurred during the neonatal period, three-fourths were in the first week of life (Figure 2a). The first 3 days of life alone accounted for more than one-half of the total neonatal deaths (Figure 2b).
The results of the two studies whose data could not be included in the final analysis were not very different from the pooled results (Table 1).17, 24 We also pooled the results for Asia and Africa separately, but we did not find any difference in the estimates for the two regions (Supplementary Figure W1).
Cause-specific neonatal mortality
Using the search strategy described above, we identified 13 studies that reported cause-specific mortality by day of life (Table 2).7, 9, 12, 13, 14, 15, 17, 18, 19, 20, 21, 22, 25 We used the data from 7 prospective studies to obtain the estimated proportions for each of the specified time points.7, 9, 12, 13, 14, 18, 19 Figures 3a–d depict the distribution of neonatal deaths caused by asphyxia, preterm birth complications, sepsis and congenital malformations, respectively. Almost all studies had assigned the cause of death as per the WHO VA algorithm.
Asphyxia
Almost all deaths (98.2%) due to asphyxia occurred in the first week of life (Figure 3a). The first day (day 0) alone contributed about three-fourths of the total asphyxia-related deaths. Less than 2% of these deaths occurred after 72 h of birth.
Preterm birth complications
More than four-fifths of deaths due to prematurity (83.2%) occurred in the first week of life; the first day (day 0) contributed around 40%. About 8 to 10% of deaths secondary to prematurity occurred in week 2 and weeks 3 to 4 of life (Figure 3b).
Sepsis
Less than one-half of the total deaths secondary to sepsis occurred in the first week of life (Figure 3c). About 30% of these deaths occurred in the second week of life and around one-fourth in weeks 3 to 4. The distribution of deaths is almost equal in each of the first 7 days after birth.
Malformations
The distribution of deaths due to malformations almost mimicked that of deaths due to prematurity. About four-fifths (78.4%) occurred in the first week of life, with the first day (day 0) contributing about 40% (Figure 3d).
Data from another six studies could not be pooled because they were incomplete (that is, available for only a few time points).15, 17, 20, 21, 22, 25 However, the results of these studies did not differ greatly from the pooled results. In subgroup analysis based on the location of countries (Asia or Africa), we did not find any difference in the estimates (Supplementary Figure W1).
Discussion
We have attempted to provide the approximate breakdown of deaths at different time points in the first 4 weeks of life based on the literature published in the last 10 years. The results are not entirely new, but tend to reaffirm the key findings of earlier reports from international agencies. More importantly, this review provides a summary of the fraction of deaths occurring at each time point in the neonatal period, which should help policy-makers and program managers devise optimal strategies for the delivery of proven interventions.
The three major findings of this review with implications for different stakeholders are the following: (1) the high proportion of neonatal deaths in the first 3 days of life, and in particular, the first day; (2) the majority of deaths due to preterm birth complications and malformations occurring in the first week; and (3) the occurrence of about one-half of deaths secondary to sepsis/pneumonia after the first week of life.
High proportion of deaths in the first 3 days of life
More than one-half of neonatal deaths (57%) occur in the first 3 days after birth. Two-thirds of these deaths occur on the first day, that is, within 24 h of birth. Neonatal deaths constitute 52% of the total under-five child deaths in WHO’s South–East Asia Region,28 and about one-third occur in the first 3 days of life. In WHO’s African Region, where neonatal deaths constitute 30% of under-five deaths,28 about 17% occur in this time period. Obviously, Millennium Development Goal 4 cannot be achieved without addressing these deaths.
Reducing mortality in the first few days after birth calls for scaling-up of evidence-based interventions beginning in the antenatal period. Unfortunately, coverage of most interventions during pregnancy and birth is less than optimal in many low- and middle-income country settings. The median coverage of two key interventions—at least four antenatal visits and presence of a skilled birth attendant at delivery—is about 50% in the 68 countdown countries.29 The coverage of postnatal visits within 2 days of birth for babies born outside a health facility is even lower, ranging from 2 to 19%, in those countries for which data are available.29 Notwithstanding the huge variation in coverage figures between the individual countries, the median data do underscore the need for coordinated efforts aimed at improving the quality of antenatal care, promoting institutional deliveries, providing optimal basic and emergency obstetric care, and ensuring immediate postnatal care of neonates in these settings.
In the last few years, many countries have made substantial progress in achieving high coverage rates for some interventions. The most striking example is the coverage of skilled care at birth and institutional deliveries. However, these gains have not resulted in the expected magnitude of benefit in terms of reduction in NMR or early NMR (that is, mortality in the first week) in many settings,30 the possible reasons being suboptimal quality of care provided in health facilities and lack of a concomitant increase in the coverage of other key interventions in the continuum of care. For example, India witnessed a large rise in institutional delivery rates after the introduction of a conditional cash transfer scheme under the National Rural Health Mission (NRHM) in mid-2005. Despite this, the average annual rate reduction of early NMR showed only a marginal change from 1.9% in the pre-NRHM era (1990 to 2005) to 2.3% after the introduction of NRHM. In contrast, the average annual reduction of late NMR and the post-neonatal infant mortality rate increased by almost threefold from the pre-NRHM to the post-NRHM period (Supplementary Figure W2). Not surprisingly, the magnitude of reduction in India’s NMR is not commensurate with that of its infant mortality rate in the last 5 years.31
Deaths due to prematurity and malformations in the first week of life
Although it is understandable that most deaths due to asphyxia occur in the first week of life, our study results indicate that even deaths secondary to preterm birth complications occur predominantly in this time period. Upon analyzing the contribution of different causes of deaths to total deaths in the first 7 days of life, we found that prematurity-related deaths accounted for almost one-third of the deaths on any given day in the first week, whereas deaths due to asphyxia peak on day 1 but diminish with each passing day (Supplementary Figure W3). This finding implies that the focus of delivery of priority interventions to reduce prematurity-related deaths should not only be on the antenatal and delivery periods but also on the first few days after birth.
Among the various interventions with the potential to reduce mortality due to preterm birth, two postnatal interventions—full supportive care for serious illness and kangaroo mother care in the facility—are estimated to have the highest impact.32 Facility-based care of premature and/or small babies born either at home or in a primary health-care facility needs to be strengthened on an urgent basis. Without ensuring optimal quality of care for these babies in specialized newborn care units, it would be almost impossible to make a significant dent in the proportion of prematurity-related deaths. Concomitantly, efforts should be made to increase the coverage of prenatal interventions such as administration of antenatal steroids to pregnant women with threatened preterm labor.
As observed with prematurity, the majority of deaths due to malformations occur in the first week of life. The malformations may have been serious or less critical, but resulted in deaths because of the delay in recognition and lack of optimal treatment facilities. Unlike deaths due to preterm birth complications, malformation-related deaths may not be reduced by simply strengthening facility-based care at the district or subdistrict level; they require establishment of quality referral institutions and ensuring optimal transport services to and from these centers. In most developing country settings, it might not be feasible to strengthen these mechanisms in the short term.
Sepsis deaths after the first week of life
About one-half of the deaths due to sepsis occurred after the first week of life. Indeed, the contribution of sepsis deaths to total deaths gradually increased from day 0 to day 6 (Supplementary Figure W3). One possible explanation for this finding is that high rates of tetanus deaths, which usually occur after the first few days of life, inflated the figures of sepsis deaths in the second and third weeks of life. However, given the small number of tetanus deaths in the studies included in this review, this explanation seems unlikely (Table 2). Another possibility is the risk of misclassifying deaths due to prematurity or other causes that occurred after the first week of life as sepsis deaths, as most studies used a VA instrument to determine the cause of deaths.
Notwithstanding the possible risk of misclassification, the results indicate that postnatal care of newborn babies—at home or in the facility—is crucial for reducing deaths due to sepsis. In addition to ensuring clean practices at birth, efforts should be made to improve the coverage of key postnatal interventions such as community-based newborn care and case management of babies with sepsis or pneumonia in the facility/community.32 More importantly, there is a need to extend the interventions beyond the first week of life—for example, ensuring at least one postnatal contact between mother–infant pairs and health-care providers in the second week of life. This contact would help to reinforce exclusive breastfeeding and cord-care practices, as well as facilitating early identification of babies at higher risk of developing sepsis (for example, those with omphalitis) and arranging timely referral to a facility for management of severe infections.
Implications for policy-makers and program managers
The findings of this review have at least three major implications for policy-makers and other key stakeholders in newborn and child health. First, there is an urgent need to improve the coverage—and more importantly the quality—of emergency obstetric care of pregnant women in health facilities. The benefits one would expect with increasing coverage of skilled delivery in most countries cannot be realized without ensuring quality care at birth. Second, the facility-based care of neonates should be strengthened to improve the care of premature and small babies in the first few days of life. This would entail establishment of more special care newborn units, allocating more resources including skilled manpower and finances to existing facilities, ensuring quality of care by implementing standardized treatment protocols and creating mechanisms to transfer such babies from the community to the facility. Third, the focus of postnatal care should not be restricted to the first week of life alone, particularly in settings with high NMR and a high proportion of sepsis deaths. Postnatal contacts between the health-care providers and the family—in the form of home visits or other mechanisms—should be extended to the second week and, if possible, beyond that.
Strengths and limitations
Ours is possibly the first attempt to systematically review the available literature for evaluating the exact timing of overall and cause-specific deaths in the neonatal period in low- and middle-income country settings. All the studies that provided data for the cause-specific deaths were prospective in nature, which reduces the risk of misclassification between stillbirths and neonatal deaths (cf. the estimates from Demographic and Health Survey data sets).33, 34
This review has some major limitations. First, the number of included studies and the number of deaths in those studies (8316 and 1781, respectively, for overall and cause-specific mortality) are relatively small. Second, five out of seven included studies used a VA instrument to assign the causes of death. The diagnostic accuracy of VA has been found to be poor for neonatal deaths.35, 36, 37 Moreover, the accuracy varies with the VA tool used, its administration, coding and classification of deaths, and cause-specific mortality fractions in a particular area.38 However, a recently published study from South–East Asia showed a reasonably high accuracy—78 to 92%—of the WHO VA instrument in ascertaining different underlying causes of death.38 Third, we had to extrapolate the data of one or more time points for a few studies based on the pooled estimates of other studies, which is likely to have introduced some amount of bias in the final estimates.
Conclusion
The first 3 days of life account for almost 60% of total neonatal deaths while the first week accounts for almost all asphyxia-related deaths and the majority of prematurity- and malformation-related deaths. About one-half of sepsis-related deaths occur after the first week. These findings indicate the need to improve the quality of care at birth and in the immediate postnatal period, to strengthen the facility-based care of small babies and to extend the scope of postnatal care beyond the first week of life.
References
Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385: 430–440.
Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 2010; 375: 1969–1987.
Lawn JE, Cousens S, Zupan J . Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? where? why? Lancet 2005; 365: 891–900.
Lawn JE, Wilczynska-Ketende K, Cousens SN . Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol 2006; 35: 706–718.
United States Agency for International Development. List of developing countries—USAID. Available at http://www.usaid.gov/sites/default/files/documents/1876/310maa.pdf (accessed 6 May 2015).
Sterne JAC, Bradburn MJ, Egger M . Meta-analysis in STATA TM. In: Egger M, Smith GD, Altman DG (eds). Systematic Reviews in Health Care: Meta-analysis in Context. BMJ Publishing: London, UK, 2001, pp 347–369.
Baqui AH, Darmstadt GL, Williams EK, Kumar V, Kiran TU, Panwar D et al. Rates, timing and causes of neonatal deaths in rural India: implications for neonatal health programmes. Bull World Health Organ 2006; 84: 706–713.
Welaga P, Moyer CA, Aborigo R, Adongo P, Williams J, Hodgson A et al. Why are babies dying in the first month after birth? A 7-year study of neonatal mortality in northern Ghana. PLoS One 2013; 8: e58924.
Nga NT, Hoa DT, Malqvist M, Persson LA, Ewald U . Causes of neonatal death: results from NeoKIP community-based trial in Quang Ninh province, Vietnam. Acta Paediatr 2012; 101: 368–373.
Belizán JM, McClure EM, Goudar SS, Pasha O, Esamai F, Patel A et al. Neonatal death in low- to middle-income countries: a global network study. Am J Perinatol 2012; 29: 649–656.
Khatun F, Rasheed S, Moran AC, Alam AM, Shomik MS, Sultana M et al. Causes of neonatal and maternal deaths in Dhaka slums: implications for service delivery. BMC Public Health 2012; 12: 84.
Bang AT, Paul VK, Reddy HM, Baitule SB . Why do neonates die in rural Gadchiroli, India? (Part I): primary causes of death assigned by neonatologist based on prospectively observed records. J Perinatol 2005; 25 (Suppl 1): S29–S34.
Edmond KM, Quigley MA, Zandoh C, Danso S, Hurt C, Owusu Agyei S et al. Aetiology of stillbirths and neonatal deaths in rural Ghana: implications for health programming in developing countries. Paediatr Perinat Epidemiol 2008; 22: 430–437.
Waiswa P, Kallander K, Peterson S, Tomson G, Pariyo GW . Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health 2010; 15: 964–972.
Bapat U, Alcock G, More NS, Das S, Joshi W, Osrin D . Stillbirths and newborn deaths in slum settlements in Mumbai, India: a prospective verbal autopsy study. BMC Pregnancy Childbirth 2012; 12: 39.
Niswade A, Zodpey SP, Ughade S, Bangdiwala SI . Neonatal morbidity and mortality in tribal and rural communities in central India. Indian J Community Med 2011; 36: 150–158.
Gill CJ, Phiri-Mazala G, Guerina NG, Kasimba J, Mulenga C, MacLeod WB et al. Effect of training traditional birth attendants on neonatal mortality (Lufwanyama Neonatal Survival Project): randomised controlled study. BMJ 2011; 342: d346.
Jehan I, Harris H, Salat S, Zeb A, Mobeen N, Pasha O et al. Neonatal mortality, risk factors and causes: a prospective population-based cohort study in urban Pakistan. Bull World Health Organ 2009; 87: 130–138.
Khanal S, Gc VS, Dawson P, Houston R . Verbal autopsy to ascertain causes of neonatal deaths in a community setting: a study from Morang, Nepal. J Nepal Med Assoc 2011; 51: 21–27.
Ngoc NT, Merialdi M, Abdel-Aleem H, Carroli G, Purwar M, Zavaleta N et al. Causes of stillbirths and early neonatal deaths: data from 7993 pregnancies in six developing countries. Bull World Health Organ 2006; 84: 699–705.
Kalter HD, Khazen RR, Barghouthi M, Odeh M . Prospective community-based cluster census and case-control study of stillbirths and neonatal deaths in the West Bank and Gaza Strip. Paediatr Perinat Epidemiol 2008; 22: 321–333.
Chowdhury HR, Thompson S, Ali M, Alam N, Yunus M, Streatfield PK . Causes of neonatal deaths in a rural subdistrict of Bangladesh: implications for intervention. J Health Popul Nutr 2010; 28: 375–382.
Campbell O, Gipson R, el-Mohandes A, Issa AH, Matta N, Mansour E et al. The Egypt National Perinatal/Neonatal Mortality Study 2000. J Perinatol 2004; 24: 284–289.
Rajindrajith S, Mettananda S, Adihetti D, Goonawardana R, Devanarayana NM . Neonatal mortality in Sri Lanka: timing, causes and distribution. J Matern Fetal Neonatal Med 2009; 22: 791–796.
Baiden F, Hodgson A, Adjuik M, Adongo P, Ayaga B, Binka F . Trend and causes of neonatal mortality in the Kassena-Nankana district of northern Ghana, 1995-2002. Trop Med Int Health 2006; 11: 532–539.
ICMR Young Infant Study Group. Age profile of neonatal deaths. Indian Pediatr 2008; 45: 991–994.
Upadhyay RP, Rai SK, Krishnan A . Using three delays model to understand the social factors responsible for neonatal deaths in rural Haryana, India. J Trop Pediatr 2013; 59: 100–105.
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 2012; 379: 2151–2161.
Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J et al. Countdown to 2015 decade report (2000-10): taking stock of maternal, newborn, and child survival. Lancet 2010; 375: 2032–2044.
Lim SS, Dandona L, Hoisington JA, James SL, Hogan MC, Gakidou E . India's Janani Suraksha Yojana, a conditional cash transfer programme to increase births in health facilities: an impact evaluation. Lancet 2010; 375: 2009–2023.
Registrar General of India Sample Registration System (SRS) Statistical Report 2010. Registrar General of India: New Delhi, India, 2012.
Friberg IK, Bhutta ZA, Darmstadt GL, Bang A, Cousens S, Baqui AH et al. Comparing modelled predictions of neonatal mortality impacts using LiST with observed results of community-based intervention trials in South Asia. Int J Epidemiol 2010; 39 (Suppl 1): i11–i20.
Kramer MS, Liu S, Luo Z, Yuan H, Platt RW, Joseph KS . Analysis of perinatal mortality and its components: time for a change? Am J Epidemiol 2002; 156: 493–497.
Lumbiganon P, Panamonta M, Laopaiboon M, Pothinam S, Patithat N . Why are Thai official perinatal and infant mortality rates so low? Int J Epidemiol 1990; 19: 997–1000.
Kalter HD, Hossain M, Burnham G, Khan NZ, Saha SK, Ali MA et al. Validation of caregiver interviews to diagnose common causes of severe neonatal illness. Paediatr Perinat Epidemiol 1999; 13: 99–113.
Marsh DR, Sadruddin S, Fikree FF, Krishnan C, Darmstadt GL . Validation of verbal autopsy to determine the cause of 137 neonatal deaths in Karachi, Pakistan. Paediatr Perinat Epidemiol 2003; 17: 132–142.
Freeman JV, Christian P, Khatry SK, Adhikari RK, LeClerq SC, Katz J et al. Evaluation of neonatal verbal autopsy using physician review versus algorithm-based cause-of-death assignment in rural Nepal. Paediatr Perinat Epidemiol 2005; 19: 323–331.
Aggarwal AK, Kumar P, Pandit S, Kumar R . Accuracy of WHO verbal autopsy tool in determining major causes of neonatal deaths in India. PLoS One 2013; 8: e54865.
Acknowledgements
We thank Dr Rajiv Bahl, Newborn Health Lead, Department of Maternal, Newborn, Child and Adolescent Health, WHO, Geneva, Switzerland, for his highly valuable comments on the methodology of the review. The Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland, funded this review. This supplement was funded by the Department of Maternal, Newborn, Child and Adolescent Health and Development, World Health Organization, Geneva, Switzerland.
Author contributions
MJS guided the development of the study protocol, searched the databases, extracted data, performed the statistical analysis and wrote the manuscript. CKN prepared the protocol, applied the search strategy, retrieved the articles, extracted data and made the initial tables. RRD retrieved the articles, extracted data and wrote the first draft of the paper. AC updated the search, retrieved the articles, extracted data and revised the final manuscript. RA modified the study protocol, supervised data extraction and modified the final version of the manuscript. VKP guided development of the protocol, helped in statistical analysis and finalized the draft manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Perinatology website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Sankar, M., Natarajan, C., Das, R. et al. When do newborns die? A systematic review of timing of overall and cause-specific neonatal deaths in developing countries. J Perinatol 36 (Suppl 1), S1–S11 (2016). https://doi.org/10.1038/jp.2016.27
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.27
This article is cited by
-
Incidence and determinants of perinatal mortality in five urban hospitals in Dar es Salaam, Tanzania: a cohort study with an embedded case–control analysis
BMC Pregnancy and Childbirth (2024)
-
A descriptive analysis of the coverage of newborn care services among women who delivered in health facilities in 17 sub-Saharan African countries
BMC Pregnancy and Childbirth (2023)
-
To what extent is antenatal care in public health facilities associated with delivery in public health facilities? Findings from a cross-section of women who had facility deliveries in Nigeria
BMC Public Health (2023)
-
Incidence and predictors of mortality among neonates admitted with perinatal asphyxia at west Oromia tertiary hospitals, Ethiopia, 2022
BMC Pediatrics (2023)
-
Determinants of early neonatal mortality (hospital based retrospective cohort study in Somali region of Ethiopia)
Scientific Reports (2023)