Abstract
Objective:
To study the impact of integrated evaluation of hemodynamics (IEH) using targeted neonatal echocardiography, together with regional tissue oxygenation, fractional oxygen extraction using near-infrared spectroscopy on the management of infants with compromised hemodynamics.
Study Design:
Retrospective cohort comparison of two groups of infants with compromised hemodynamics. EPOCH 1: did not undergo IEH (January 2012 to March 2014); EPOCH 2: underwent IEH (April 2014 to December 2015). The primary outcome was the time to recovery.
Results:
In all, 340 infants were included; 158 underwent IEH with a median (IQR) of 2 (1 to 3) evaluations per infant. Reasons for assessment included PDA (60%), compromised systemic circulation (14%) and clinically suspected pulmonary hypertension (22%). The time to recovery was shorter in IEH group in patients with compromised systemic circulation median (IQR), 32 h (24 to 63) compared with none IEH group 71 h (36 to 96), pulmonary hypertension 63 h (14.2 to 102) in IEH group compared with 68 h (24 to 240) in none IEH group, there were fewer PDA-related complications in preterm infants with PDA in IEH group.
Conclusion:
IEH was associated with shorter time to clinical recovery in infants with compromised hemodynamics
Similar content being viewed by others
Introduction
Intact or normal hemodynamics implies blood flow that provides adequate oxygen and nutrient delivery to the tissues.1 Blood flow varies with vascular resistance and cardiac function; both may be reflected in blood pressure.2 Normal cardiovascular dynamics should be considered within the context of global hemodynamic function, with the aim of achieving normal oxygen delivery and end-organ performance.3 The current routine assessment of hemodynamics in sick preterm and term infants is based on incomplete information. We have addressed this by adopting an approach utilizing objective techniques, namely integrating targeted neonatal echocardiography (TNE) with near-infrared spectroscopy (NIRS). Implementation of these techniques requires an individual with the requisite TNE training, preferably in an accredited program, who also has a good understanding of perinatal and neonatal cardiovascular, respiratory and other specific end-organ physiology.
Determining a neonate’s capacity to autoregulate is crucial in integrated evaluation of hemodynamics (IEH). In this study, we describe the impact of integrating neonatal hemodynamics using TNE with indices of tissue oxygenation within a tertiary neonatal intensive care unit. We have defined ‘intact hemodynamics physiology’ as the integrated ability of the cardiovascular system, the oxygen-carrying capacity of the blood and autoregulatory mechanisms to be adequate for normal end-organ function.4 TNE uses ultrasound to longitudinally assess myocardial performance, systemic and pulmonary blood flow, as well as identifying intracardiac and extracardiac shunts.5 It provides detailed real-time information concerning cardiovascular physiology, which may lead to rapid identification of the pathophysiology of circulatory failure in critically ill neonates such as those with sepsis or persistent pulmonary hypertension.6 NIRS cannot diagnose specific pathophysiology, but it can alert the health-care team to early changes in tissue oxygenation.7 Abnormal NIRS readings may reflect other confounders that affect oxygen delivery such as hypocapnia, high mean airway pressure, low hemoglobin or a hemodynamically significant PDA; all of these confounders must be assessed when considering our integrated model and before relying on NIRS as a surrogate marker for blood flow.8
By integrating information from the clinical examination, NIRS, and TNE a complete picture can be obtained.4 Autoregulation involves local vasodilation through the release of nitric oxide and other vasodilators. When autoregulation is compromised, there is an increase in tissue oxygen extraction.9 Thus increased fractional oxygen extraction (FOE) can be regarded as a compensatory step before the maximum oxygen extraction capacity being reached resulting in ensuing lactic acidosis.10
The purpose of this study reports is to report preliminary results of the implementation of IEH in an neonatal intensive care unit and to examine whether IEH can improve the outcome of newborn infants with compromised hemodynamics.
Methods
Institutional ethics board approval was obtained before commencing this retrospective study of all infants with compromised hemodynamics admitted to the two neonatal intensive care units in Winnipeg between January 2012 and December 2015. Retrospective chart review and hemodynamics consultation records were used to identify all infants who underwent IEH by a trained neonatologist11 from April 2014 to December 2015 (EPOCH 2). Pharmacy’s electronic records of infants who received cardiovascular medications and section of respiratory therapy’s records of infants who received respiratory support and/or inhaled nitric oxide (iNO) were also reviewed. Similar chart and record review was made of all infants admitted with similar clinical conditions—as per inclusion criteria—between January 2012 to March 2014 (EPOCH 1) and who had not received IEH.
The decision to request IEH consultation in sick infants with suspected compromised hemodynamics was made by the clinical team. Figure 1 shows the flow charts with included and excluded infants for the three clinical categories from both EPOCHS.
IEH is based on evaluation of components contributing to blood flow and tissue oxygenation as follows (Figure 2):
Clinical evaluation
Either Clinical evidence of compromised systemic circulation with at least one of the followings: low mean blood pressure (MABP) (less than the lower 95th confidence interval for corrected gestational age),12 oliguria <1 ml kg−1 h−1 for at least 12 h beyond first 12 h after birth, or lactic acidosis >2.8 mmol dl−1, or clinical evidence of worsening hypoxemia defined as increased fractional inspired oxygen by 0.1 above baseline for more than 24 h.
Targeted neonatal echocardiography
This was performed using either vivid.i or vivid-E9 echocardiography machine (GE, Waukesha, WI, USA). Myocardial performance, systemic and pulmonary blood flow and intra- and extra-cardiac shunts were assessed. A review by a pediatric cardiologist for structural abnormalities was performed for all initial echocardiographic scans.
Tissue oxygenation
Arterial oxygen saturation was measured by a pulse oximeter; regional tissue oxygenation (RTO) by NIRS, and FOE is the calculated ratio between oxygen delivery and extraction.13 RTO and FOE were never used in isolation from the integrated approach for decision making. RTO was performed to monitor either cerebral oxygenation alone or both cerebral (crSO2) and mesenteric (mrSO2), using NIRS8 (FORE-SIGHT Absolute Tissue Monitor, Casmed, Branford, CT or NONIN medical’s senSmart oximetry Modelx-100).8, 10, 14, 15, 16
Cerebral or mesenteric autoregulation
We considered cerebral or mesenteric autoregulation to be compromised if RTO<55% and FOE>0.33 for at least 10 min, together with either low mean blood pressure or TNE evidence of compromised systemic circulation (low cardiac output <150 ml kg−1 min due to poor heart filling or poor myocardial performance, or abnormal diastolic flow pattern in one or both the mesenteric or middle cerebral arteries), RTO should be considered low after correction of hypocarbia (PCO2 <40 mm Hg) and low hemoglobin <100 g l−1 as per our unit guidelines. Figure 3 shows stages of compromised systemic circulation including compensatory role of autoregulation.
IEH report and recommendation
Following completion of the comprehensive IEH, findings were summarized in two different reports, one for TNE findings with no recommendations included and another detailed IEH report with all calculations, current support and preliminary suggested plan by the clinical team, pathophysiologic impression of the IEH physician clearly formulated recommendations and plan for follow-up. These plans could include initiation, weaning or discontinuation of medications, adjustment of ventilator settings, or decision for ECMO support. Subsequent, follow-up was also documented, together with the level of response.
Data collection
Patient demographics and baseline characteristics collected were birth weight, gestational age, postnatal age, perinatal history and gender; clinical data, detailed clinical respiratory and cardiovascular parameters, mode of respiratory support and cardiovascular medications, recorded either until recovery or death. Evaluation of NIRS monitoring was considered only in those infants with suspected compromised systemic blood flow or worsening of hypoxemia and was recorded at the same time as the TNE. NIRS monitoring was continued up to 10 days as per the recommendations of the IEH consultant and according to the clinical circumstances.
The primary outcome was the time from the start of clinical deterioration to clinical recovery as defined by normalization of blood pressure, recovery from oliguria and lactic acidosis, and commencement of successful weaning of inotropes, vasopressor, pulmonary vasodilators or oxygen. The outcome in preterm infants with PDA was the rate of PDA ligations and post PDA ligation syndrome (EPOCH 2) compared with infants admitted with similar conditions and managed without being assessed by IEH (EPOCH 1).
We graded the impact of the formulated medical recommendation as follows:
Grade 1: Clinical recovery within 72 h after IEH assessment and recommendations.
Grade 2: Avoidance or discontinuation of intervention plan proposed by the clinical team prior to IEH or discontinued as recommended by IEH team and associated with short-term clinical improvement within 72 h
Grade 3: Improvement after 72 h from the acute clinical condition as above
Grade 4: No change of clinical team’s plan, with short-term improvement within 72 h
Grade 5: Worsening of the clinical condition after IEH new recommendation
Grades 1, 2 and 4 were considered positive; grade 3 was considered undetermined or potentially positive, and grade 5 was considered to be negative. This grading was based on information obtained from the IEH consult form and daily follow-up by the IEH physician and neonatal team on service and documented in the chart.
We compared the clinical characteristics of all included infants in both EPOCHS at 6 h after clinical deterioration. This was before IEH consultation in all infants with compromised systemic circulation or pulmonary hypertension. We compared clinical parameters at 48 h after clinical deterioration in both EPOCHS to determine improvement or otherwise after the first stage of hypoxia or energy failure. This was after IEH consultation in all infants in EPOCH 2. Seventy-two hours was considered the cutoff for global clinical recovery. This time was chosen to exclude deterioration due to reperfusion injury after initial improvement.17
Statistical analysis
SPSS v. 24 (SPSS, Chicago, IL, USA) was used to perform the statistical analysis. Data are presented as median with interquartile range or frequencies. Comparisons between groups were analyzed by Mann–Whitney U-test; frequencies were analyzed using χ2; P<0.05 was considered significant. Multivariate and adjusted multivariable analysis between groups was also performed using the general linear model to test effects between subjects. The primary outcome for infants with compromised systemic circulation was evaluated using Cox proportional hazards regression model.
Results
Retrospective chart review identified 182 infants in EPOCH 1 and 158 infants in EPOCH 2, the latter resulting in 367 IEH assessments. Gestation age was 29 (26.2, 35.5) and 28.5 (26.3, 34.2) weeks, and birth weight 1185 (703, 2213) and 1274 (795, 2157)g, for EPOCH 1 and EPOCH 2, respectively. Initial IEH assessment was performed at the postnatal age of 13 (8, 49) days. Each infant underwent 2 (IQR 1–3) evaluations.
Indications for IEH consultation included PDA before and after either medical (indomethacin or ibuprofen) or surgical PDA treatment: 77 patients (216 studies, 60%). Clinically suspected compromised systemic blood flow in term and preterm infants: 27 patients (51 studies, 14%). Infants with clinically suspected pulmonary hypertension or worsening of hypoxemia: 45 patients (84 studies, 22%). Other miscellaneous indications included the checking of a central line for abnormal placement with suspected migration and pericardial effusion: nine patients (16 studies, 4%). The pathophysiologic correlates with the clinical indications and associated TNE and NIRS findings are listed in Table 1. Some cases showed both compromised systemic circulation with either pulmonary overcirculation or pulmonary hypertension present, explaining the overlap. Table 2 shows baseline and clinical characteristics for the two EPOCHs comparing the three clinical conditions.
Infants with compromised systemic circulation
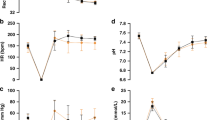
There was no significant difference between the two EPOCHS in infants with compromised systemic circulation regarding initial baseline characteristics. At 48 h MABP was significantly higher, and lactic acid was significantly lower in patients of EPOCH 2. Time to clinical recovery was significantly less in patients of EPOCH 2. Mortality rates due to shock were not significantly different between the EPOCHS. There was a highly significant difference between the two EPOCHS when considered jointly (multivariate analysis) with Wilk’s Lambda= 0.5, P=0.0001 partial ŋ2 =0.48. A separate ANOVA was conducted for each dependent variable, with each ANOVA evaluated at an α-level of 0.025, there was a significant difference at 48 h between the two EPOCHS in lactic acid (P=0.008, partial ŋ2=0.13), MABP (P=0.001, partial ŋ2=0.25) and time to clinical recovery (P=0.012, partial ŋ2=0.126). At 6 h there was no significant difference between the two EPOCHS in lactic acid at 6 h after clinical deterioration (P=0.3, partial ŋ2=0.002), MABP (P=0.84, partial ŋ2=0.001) and mortality rate due to shock (P=0.13, partial ŋ2=0.046). In a mutivariable analysis, including adjustment for confirmed infection and gestational age, the time to clinical recovery remained significantly lower in EPOCH 2, mean 30.6 h (95% CI, 13 to 59.8) compared with EPOCH 1, mean, 96.5 (95% CI, 73.4 to 114.5) (P=0.002). In another mutivariable analysis using Cox regretion model including adjustment for basline blood pressure (mean, systolic and diastolic), lactic acid, urine output, the use of IEH in EPOCH 2 was independently associated with reduced time to clinical recovery (cause-specific HR, 0.3 (95% CI, 0.14 to 0.6) P=0.002; gestational age was not independntly associated with increased time to clinical recovery (cause-specific HR 1.16 (95% CI, 0.9 to 1.5) P=0.23, and confirmed infection was not independently associated with increaed time to clinical recovery (cause-specific HR, 0.38 (95% CI, 0.24 to 1.2) P=0.062. For infants with compromised systemic circulation, time to clinical recovery using Cox proportional hazards is compared for EPOCHS 1 and 2 (P=0.002) (Figure 4).
Preterm infants with PDA
The rates of PDA ligation and associated post PDA ligation syndrome were significantly lower in EPOCH 2. There was a significant difference between the two EPOCHS when considered jointly (multivariate analysis) with Wilk’s Lambda= 0.8, P=0.0001 and partial ŋ2=0.17. A separate ANOVA was conducted as described before for each variable. There was a significant difference between the two EPOCH on the rate of PDA ligation (P=0.007, partial ŋ2=0.043), post PDA ligation syndrome (P=0.02, partial ŋ2=0.032) and rate of follow-up (P=0.0001, partial ŋ2=0.1).
Term and near-term infants with pulmonary hypertension
Oxygenation index (OI) at 48 h after clinical deterioration was significantly lower in term infants from EPOCH 2 with pulmonary hypertension; time to recovery was significantly less, and fewer cases were unresponsive to iNO.
There was a significant difference between the two EPOCHS when considered jointly (multivariate analysis) with Wilk’s Lambda= 0.84, P=0.006 and partial ŋ2=0.15. A separate ANOVA was conducted as described before for each variable. There was a significant difference between the two EPOCHS on OI 48 h after clinical deterioration (P=0.001, partial ŋ2=0.8), time to clinical recovery (P=0.013, partial ŋ2=0.56), but there was no significant difference on unresponsiveness to iNO (P=0.06, partial ŋ2=0.031) and OI 6 h after clinical deterioration.
The graded impact of IEH assessment on clinical management is shown in Table 3. The improvement was documented within 72 h in 40% of infants after change of management plan; 19% after discontinuation of a planned or started an intervention, 20% with support of current management in (i.e. a total of 79% improved within 72 h after IEH), undetermined outcome including infants with documented improvement after 72 h in 15% (potential positive outcome) and negative in 1%. Three patients continued to deteriorate with irreversible shock despite implementing IEH recommendation; two were surgical patients with bowel ischemia demonstrated either by postmortem pathology or open surgery, probably due to reperfusion injury. Table 3 also shows the number of infants with compromised autoregulation in each group. It is difficult to apply the same graded impact of regular clinical care on infants from EPOCH 1, but we have documented improvement shortly after the start of the management plan (within 72 h) in 33.5% of the studied group.
Discussion
There is increasing recognition by neonatologists that the focus of monitoring systems must change from those of solely blood pressure and arterial oxygen saturation monitoring to those that measure organ perfusion and tissue oxygen delivery.10 Current research confirms that the measurement of blood pressure does not reflect either tissue perfusion or oxygen delivery,18, 19 and current therapeutic interventions for hypotension are not associated with improved in-hospital outcomes or neurodevelopmental outcomes.20, 21 Objective evaluation of hemodynamics using the newer techniques of TNE and NIRS have proven to be valuable in evaluation of systemic blood flow, pulmonary blood flow, myocardial performance and tissue oxygen delivery; integrating TNE findings, NIRS and clinical parameters can reflect the integrity of autoregulatory mechanisms.10, 15 Recent research highlighted the beneficial use of coherence analysis of MABP and NIRS to predict changes in autoregulation in sick neonates.10, 22, 23 This new approach has the potential to positively impact decision-making and optimize organ performance in vulnerable neonates 2425
We have demonstrated a significantly shorter time to clinical recovery; although mortality rate was lower this was nonsignificant. We were able to determine that autoregulation was compromised in many cases either in the brain or mesenteric region; this was helpful to target treatment, monitor the response, and limit progression to tissue hypoxia and organ dysfunction. On the other hand, it avoided treating infants with borderline or fluctuant blood pressure when brain RTO and FOE were normal, and this contributed to the outcome of avoidance or discontinuation of unnecessary planned interventions.15 There is support recently for adopting a comprehensive approach to identifying and treating compromised end-organ oxygen delivery, that is, individualizing patient care.26 Recent advances in NIRS combined with the use of TNE; continuous blood pressure monitoring and periodic assessments of indirect signs of tissue perfusion can finally enable us to obtain a more holistic idea of compromised neonatal hemodynamics.27 Anderliesten et al. concluded that MABP less than GA was not associated with lower brain TOI or with lower neurodevelopmental outcome scores. Importantly, regardless of MABP, low brain TOI was associated with lower neurodevelopmental outcome scores.10
Our study together with other recent publications highlights the importance of considering parameters reflecting blood flow and oxygen delivery.28 The same principle was considered in preterm infants with PDA as we deferred planned PDA ligation when mesenteric and brain RTO and FOE were normal and in whom there was no severe respiratory compromise; this may explain a lower PDA ligation rate and its related complications in EPOCH 2.29, 30 Time to clinical recovery and OI in infants with pulmonatry hypertension were significantly lower in infants of EPOCH 2 with less reported cases unresponsive to iNO in infant of EPOCH 2, which may explain the usefulness of TNE in early real-time assessment of right ventricle dysfunction, low preload as common causes of resistance to iNO,31 and Table 3 shows selection criteria of different pulmonary vasodilators.
The decisions both to request IEH consultation and to accept the formulated medical recommendation were at the discretion of the neonatologist on service. This is the first report to our knowledge of combining the clinical use of TNE with evaluation of RTO, FOE and autoregulation by NIRS, with full interpretation and formulated recommendation by a neonatologist educated and trained in hemodynamics. The main limitation is its retrospective methodology; a further technical limitation is the difficulty and reliability of assessing mesenteric RTO and FOE by NIRS, resulting in our relying solely on cerebral NIRS values in some cases, frequent atrefacts and the presence of many confounders that can affect NIRS values, mainly PCO2 levels.32, 33
Conclusion
Application of our integrated approach has led to improved understanding of the underlying physiology in infants with compromised systemic and pulmonary circulation. It has also resulted in shorter time to clinical recovery. Further studies are needed to confirm the value of this new approach and to examine the impact of IEH on long-term outcome.
References
Wolff CB . Normal cardiac output, oxygen delivery and oxygen extraction. Adv Exp Med Biol. 2008; 599: 169–182.
Azhan A, Wong FY . Challenges in understanding the impact of blood pressure management on cerebral oxygenation in the preterm brain. Front Physiol 2012; 3: 1–8.
De Boode WP . Clinical monitoring of systemic hemodynamics in critically ill newborns. Early Hum Dev 2010; 86 (3): 137–141.
Elsayed Y, Fraser D . Integrated evaluation of neonatal hemodynamics program optimizing organ perfusion and performance in critically ill neonates, Part 1: Understanding physiology of neonatal hemodynamics. J Neonatal Netw 2016; 35 (3): 143–150.
El-Khuffash AF, McNamara PJ . Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin Fetal Neonatal Med 2011; 16 (1): 50–60.
Kluckow M, Seri I, Evans N . Functional echocardiography: an emerging clinical tool for the neonatologist. J Pediatr 2007; 150 (2): 125–130.
Pellicer A, Bravo MDC . Near-infrared spectroscopy: a methodology-focusedreview. Semin Fetal Neonatal Med 2011; 16 (1): 42–49.
Greisen G, Leung T, Wolf M . Has the time come to use near-infrared spectroscopy as a routine clinical tool in preterm infants undergoing intensive care? Philos Trans R Soc A Math Phys Eng Sci 2011; 369: 4440–4451.
Liem KD, Greisen G . Monitoring of cerebral haemodynamics in newborn infants. Early Hum Dev 2010; 86 (3): 155–158.
Alderliesten T, Lemmers PMA, Smarius JJM, Baerts W, Van Bel F . Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr 2013; 162 (4): 698–704.
Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Eur J Echocardiogr 2011; 12 (10): 715–736.
Zubrow AB(1), Hulman S, Kushner H, Falkner B . Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. J Perinatol 1995; 6: 470–479.
Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B . Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr 2013; 163: 1558–1563.
Goff Da, Buckley EM, Durduran T, Wang J, Licht DJ . Noninvasive cerebral perfusion imaging in high-risk neonates. Semin Perinatol 2010; 34 (1): 46–56.
Verhagen EA, Hummel La, Bos AF, Kooi EMW . Near-infrared spectroscopy to detect absence of cerebrovascular autoregulation in preterm infants. Clin Neurophysiol 2014; 125 (1): 47–52.
Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG . Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern Fetal Neonatal Med 2011; 24 (4): 574–582.
Gunn AJ, Gunn TR, De Haan HH, Williams CE, Gluckman PD . Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J Clin Invest 1997; 99 (2): 248–256.
Weindling M, Paize F . Peripheral haemodynamics in newborns: best practice guidelines. Early Hum Dev 2010; 86 (3): 159–165.
Weindling AM . Peripheral oxygenation and management in the perinatal period. Semin Fetal Neonatal Med 2010; 15 (4): 208–215.
Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA et al. Early blood pressure, antihypotensive therapy and outcomes at 18–22 months ’ corrected age in extremely preterm infants. Arch Dis Child Fetal Neonatal Ed 2016; 101: F201–F206.
Batton AB, Li L, Nancy S . Use of antihypotensive therapies in extremely preterm infants. J Pediatr 2013; 131 (6): e1865–e1873.
Riera J, Caba F, Serrano J, Bravo C, Laura S . New time-frequency method for cerebral autoregulation in newborns: predictive capacity for clinical outcomes. J Pediatr 2014; 165: 897–902.
Vesoulis Za, Liao SM, Trivedi SB, El Ters N, Mathur AM . A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr Res 2015; 79 (3): 453–459.
Pellicer A, Greisen G, Benders M, Claris O, Dempsey E, Fumagalli M et al. The SafeBoosC phase II randomised clinical trial: a treatment guideline for targeted near-infrared-derived cerebral tissue oxygenation versus standard treatment in extremely preterm infants. Neonatology 2013; 104 (3): 171–178.
El-Khuffash A, Herbozo C, Jain A, Lapointe A, McNamara PJ . Targeted neonatal echocardiography (TnECHO) service in a Canadian neonatal intensive care unit: a 4-year experience. J Perinatol 2013; 33: 1–4.
Soleymani S, Borzage M, Seri I . Hemodynamic monitoring in neonates: advances and challenges. J Perinatol 2010; 30 (Suppl S1): S38–S45.
Cayabyab R, McLean CW, Seri I . Definition of hypotension and assessment of hemodynamics in the preterm neonate. J Perinatol 2009; 29 (Suppl 2 (S2): S58–S62.
Noori S, Seri I . Evidence-based versus pathophysiology-based approach to diagnosis and treatment of neonatal cardiovascular compromise. Semin Fetal Neonatal Med 2015; 20 (4): 238–245.
Chock VY, Ramamoorthy C, Van Meurs KP . Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J Pediatr 2012; 160 (6): 936–942.
Lemmers PMa, Toet MC, van Bel F . Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 2008; 121 (1): 142–147.
Jain A, Mcnamara PJ . Persistent pulmonary hypertension of the newborn: physiology, hemo-dynamic assessment and novel therapies. Curr Pediatr Rev 2013; 9 (1): 55–66.
Noori S, Stavroudis Ta, Seri I . Systemic and cerebral hemodynamics during the transitional period after premature birth. Clin Perinatol 2009; 36 (4): 723–736.
Sortica C, Greisen G, Austin T . Is near-infrared spectroscopy clinically useful in the preterm infant. Arch Dis Child Fetal Neonatal Ed 2015; 100: 558–562.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Disclosure
The material is original research, has not been previously published and has not been submitted for publication elsewhere while under consideration.
Rights and permissions
About this article
Cite this article
Elsayed, Y., Amer, R. & Seshia, M. The impact of integrated evaluation of hemodynamics using targeted neonatal echocardiography with indices of tissue oxygenation: a new approach. J Perinatol 37, 527–535 (2017). https://doi.org/10.1038/jp.2016.257
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.257
This article is cited by
-
A new physiologic-based integrated algorithm in the management of neonatal hemodynamic instability
European Journal of Pediatrics (2022)
-
Point-of-care ultrasound (POCUS) protocol for systematic assessment of the crashing neonate—expert consensus statement of the international crashing neonate working group
European Journal of Pediatrics (2022)
-
End-organ saturations correlate with aortic blood flow estimates by echocardiography in the extremely premature newborn – an observational cohort study
BMC Pediatrics (2021)
-
Perioperative management of arteriovenous malformation guided by integrated evaluation of hemodynamics
European Journal of Pediatrics (2021)
-
The perfusion index histograms predict patent ductus arteriosus requiring treatment in preterm infants
European Journal of Pediatrics (2021)