Abstract
Objective:
The objective of the study was to compare the effect of two different dexamethasone regimens on respiratory outcomes of ventilator-dependent preterm infants.
Study Design:
Retrospective study of ventilated preterm infants <29 weeks gestational age treated with either 7-day or 10-day dexamethasone course. Primary outcome was days to successful extubation. Other outcomes included rate of successful extubation and need for repeat steroid therapy.
Results:
Fifty-nine infants were identified; 32 (54%) received 7 days of dexamethasone and 27 (46%) received 10 days of dexamethasone. Both groups had comparable baseline demographics and clinical characteristics. Mean time to successful extubation was similar between the two groups (5.1±2.7 days in 7-day group and 6.0±3.7 days in 10-day group, P=0.42). Successful extubation by end of treatment (56% versus 67%, P=0.44) and need for repeat steroid therapy (47% versus 33%, P=0.43) were also similar.
Conclusion:
7-day and 10-day course of dexamethasone have comparable efficacy in facilitating extubation of ventilator-dependent preterm infants.
Similar content being viewed by others
Introduction
Preterm infants who remain on prolonged mechanical ventilation are at increased risk for poor outcomes, including bronchopulmonary dysplasia (BPD), neurodevelopmental impairment and death.1 In such infants, a course of systemic steroids may be beneficial in improving lung compliance, decreasing airway resistance and facilitating weaning from mechanical ventilation.2, 3, 4, 5 Steroid treatment, however, is not without risk. Of particular concern is the increase in cerebral palsy and other adverse neurological outcomes seen in infants treated with steroids.6, 7, 8, 9
The optimal dosing and duration of treatment of steroids in preterm infants remain unknown. High-dose dexamethasone (starting dose >0.5 mg kg−1) have been shown in animal studies to result in apoptosis of progenitor cells in the developing brain.10, 11 In addition, in vitro studies using neutrophils isolated from cord blood suggest that high-dose dexamethasone was as much as five to ten times higher than what was necessary to achieve effective anti-inflammatory activity.12 Recently, randomized trials have shown that low-dose dexamethasone (starting dose ⩽0.2 mg kg−1 per day) given over 10 to 14 days remains effective in improving respiratory mechanics and facilitating extubation of ventilator-dependent preterm infants.4, 13, 14 Low-dose dexamethasone also does not appear to have many of the short-term side effects commonly seen with previous regimens that used higher doses. More importantly, there have been no reports of increased neurodevelopmental impairment associated with the use of low-dose dexamethasone in preterm infants.
Our unit has had experience with using two dosing regimens of low-dose dexamethasone: a 10-day course (total dose 0.89 mg kg−1) previously used by Doyle et al.,13 and a modified 7-day course (total dose 0.72 mg kg−1). We hypothesized that the shorter, 7-day course of dexamethasone is as effective as the longer, 10-day course in facilitating extubation. To test this hypothesis, we compared the time needed to achieve successful extubation of infants treated with either the 7-day or 10-day course of low-dose dexamethasone. We also looked at other respiratory outcomes including rate of successful extubation, rate of retreatment with steroids and rate of severe BPD.
Methods
This retrospective study took place at the neonatal intensive care unit of Children’s Mercy Kansas City, a level IV nursery in Kansas City, Missouri with more than 400 very-low birth weight infant admissions every year. The study was conducted from January 2010 to December 2015 using data from the data repository of the Center for Infant Pulmonary Disorders at Children’s Mercy Hospital, with approval from the local institutional review board.
Study population
Mechanically ventilated preterm infants <29 weeks gestational age at birth who were treated with dexamethasone for BPD using the unit’s protocol were identified from the data repository. Only infants who received dexamethasone for the first time for BPD were included in the study. Infants who previously received steroids for vasopressor-resistant hypotension or for airway edema were still considered eligible for study inclusion. Infants with multiple congenital anomalies or with congenital heart disease were excluded.
Intervention
Since 2010, our unit has adopted a protocol that standardized dosing of dexamethasone use in preterm infants to prevent or treat BPD. The protocol allowed for infants to be treated with either a 10-day or a 7-day course of dexamathasone. Infants who received the 10-day course received 0.15 mg kg−1 per day for 3 days, 0.10 mg kg−1 per day for 3 days, 0.05 mg kg−1 per day for 2 days and 0.02 mg kg−1 per day for 2 days, for a total dose of 0.89 mg kg−1. Infants who received the 7-day course received 0.15 mg kg−1 per day for 3 days, 0.10 mg kg−1 per day for 2 days, 0.05 mg kg−1 per day for 1 day and 0.02 mg kg−1 per day for 1 day, for a total dose of 0.72 mg kg−1.
The decision to treat infants with systemic steroids for BPD was based on individualized risk-benefit assessment as determined by the treating neonatologist. The selection of which dexamethasone regimen to use, as well as decisions about subsequent ventilator weaning and extubation, was also made by the treating neonatologist. Our clinical consensus is that intubated infants are weaned to maintain a pH of ⩾7.2 and a partial pressure of carbon dioxide (PCO2) of ⩽65 mm Hg on capillary blood gases. Extubation is usually attempted when ventilator rate is ⩽20 breaths per minute. Infants are routinely extubated to nasal continuous positive airway pressure or nasal intermittent positive pressure ventilation as the primary method of respiratory support post extubation, with high-flow nasal cannula reserved as step-down support when weaning from nasal continuous positive airway pressure. Reintubation is considered when (1) two consecutive blood gases have a pH<7.2 and PCO2>65 mm Hg, (2) fraction of inspired oxygen (FiO2) needed to maintain oxygen saturations ⩾90% has increased by more than 0.2 from pre-extubation levels or (3) more than one apneic episode needing bag-mask ventilation has occurred.
Outcome measures
The primary outcome of interest was days to achieve successful extubation, which was defined as extubation within 14 days of starting steroid treatment. Infants who remained intubated more than 14 days from the start of steroid treatment, or who needed reintubation within 72 h of extubation were considered to have failed extubation.
Data collection
De-identified data from the hospital medical records were recorded by trained data abstractors into the data repository. Data regarding infant demographics as well as clinical characteristics at start of dexamethasone treatment were collected. Changes in mean airway pressure (MAP) and FiO2 before and during dexamethasone treatment were also obtained. Other efficacy outcomes of interest included major respiratory outcomes, including rate of successful extubation; need for repeat steroid therapy for BPD; rate of severe BPD at 36 weeks; need for tracheostomy; total days on oxygen; and total days on mechanical ventilation.
Statistical analysis
Differences in demographic factors, baseline clinical characteristics and respiratory outcomes between infants treated with 7 days versus 10 days of dexamethasone were determined by using Fisher’s exact test, t-test, or Kruskal–Wallis test, as appropriate. For the primary outcome, an unadjusted comparison of the mean time to achieve successful extubation in both the groups was performed, and Kaplan–Meier proportional hazards modeling was used to visualize days needed for outcome of successful extubation to occur. Mixed-model repeated measures analysis of variance was used to assess differences in MAP, FiO2 and respiratory severity score (RSS=MAP × FiO2) between the two groups during steroid treatment. All statistical tests were two-sided. Statistical significance was set at a P-value of <0.05. Statistical analysis was performed using SPSS 20 and SAS 9.4 (Cary, NC, USA).
Results
Of the 59 preterm infants identified during the study period, 32 received 7-day dexamethasone and 27 received 10-day dexamethasone. The two groups were comparable in terms of baseline demographics, including birth weight, gestational age, sex and race (Table 1). Postnatal age and corrected gestational age at the time steroids were given did not differ between the two groups (Table 2). The MAP, FiO2 and PCO2 at start of treatment were also similar (Table 2).
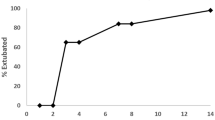
We found that 18 of 32 (56%) infants in the 7-day group and 18 of 27 (67%) infants in the 10-day group successfully extubated within 14 days of start of treatment (P=0.42). The mean time to successful extubation was similar between the two groups (5.1±2.7 days in the 7-day group and 6.0±3.7 days in the 10-day group, P=0.42, Table 3 and Figure 1). There was no difference in the type of respiratory support received post extubation between the two groups (Table 3).
The rate of requiring a second course of dexamethasone was similar in infants treated with 7-day versus 10-day dexamethasone (Table 3). This finding remained the same even when the analysis was limited to the infants who successfully extubated (3 of 18 (17%) in the 10-day group versus 6 of 18 (33%) in the 7-day group, P=0.44). There was also no difference between the two groups in the days that passed before a repeat course of dexamethasone was given (Table 3).
MAP, FiO2 and RSS decreased in both the groups during dexamethasone treatment. No substantial difference in the rates of decrease in MAP, FiO2 and RSS was found between the two groups (Figure 2). Rates of severe BPD and eventual need for tracheostomy, as well as total days on oxygen and total days on mechanical ventilation, were also similar (Table 3).
Discussion
In this retrospective study, we found no difference in the time needed to achieve successful extubation among ventilator-dependent preterm infants treated with either a 7-day dexamethasone course or a 10-day dexamethasone course. Rate of successful extubation and need for repeat steroid treatment for BPD were also similar between the two groups. Respiratory outcomes at discharge—including rate of severe BPD, total days on oxygen and total days on mechanical ventilation—were also comparable.
The optimal dosing regimen for dexamethasone—the most widely used steroid for BPD in preterm infants—has yet to be established. Because of serious adverse effects to the developing brain, earlier regimens that used high doses of dexamethasone are no longer recommended by the American Academy of Pediatrics.15 In addition, studies that directly compared high versus low doses of dexamethasone showed no additional benefit associated with the use of high-dose dexamethasone in preterm infants.16, 17, 18 Recently, randomized trials of low-dose dexamethasone versus placebo demonstrated that low-dose dexamethasone is effective in improving lung function and facilitating weaning from mechanical ventilation.4, 13, 14, 19, 20 Low-dose dexamethasone also does not appear to have the same degree of adverse effects as high-dose dexamethasone.
The lower limit at which dexamethasone remains effective has not yet been defined. In a case series of mechanically ventilated preterm infants, treatment with a course of extremely low-dose dexamethasone (total dose 0.24 mg kg−1) was associated with improvement in oxygenation index and successful extubation in 12 of the 16 infants.21 The study, however, was small and lacked appropriate controls for comparison. In another study, treatment with a low-dose dexamethasone regimen called Minidex (total dose 0.65 mg kg−1) was shown to be effective in facilitating extubation of ventilator-dependent preterm infants compared with matched controls who did not receive steroids.22 This study led to the development of a randomized trial comparing Minidex with placebo that is expected to start enrollment shortly in the United Kingdom.23
The 7-day course used in this study is a modified version of the 10-day weaning regimen used by Doyle et al.13 in the Dexamethasone: A Randomized Trial (DART) study. In designing this shorter regimen, we took into account the clinical observation that successful extubation typically occurs in the first few days of starting steroid treatment.24 We hypothesized, based on this observation, that a regimen that keeps the first part of the DART regimen the same while shortening the second part would still be effective while minimizing steroid exposure. The difference in dose between the two regimens is 0.17 mg kg−1, which is about a 19% decrease from the 10-day course.
Our study showed no difference in the rate of retreatment with steroids between infants who received 7-day versus 10-day dexamethasone. This finding is consistent with a randomized, double-blinded study by Malloy et al.19 that found no difference in the need for repeat steroid treatment between the groups treated with either high-dose or low-dose dexamethasone. Unfortunately, the study, which was only able to recruit 16 patients, was underpowered to detect meaningful differences in outcome. In another study, Jones and Davies 18retrospectively compared two different dosing regimens of dexamethasone for BPD. They found that infants treated with a shorter, 2-week regimen had higher need for repeat steroid therapy compared with infants treated with a longer, 6-week course (46% versus 22%, P=0.01), suggesting that treatment with lower doses of dexamethasone may be associated with an increased need for steroid retreatment later on.18 However, in this study, the baseline clinical characteristics between the two treatment groups were not comparable, making it difficult to ascertain whether the difference in retreatment rates was due to the different steroid regimens or due to inherent differences between the two groups.
Of the 9 infants who received tracheostomy in our study, 5 had structural airway issues, including subglottic stenosis, tracheobronchomalacia and unilateral vocal cord paralysis. Three of the 5 infants were treated with 10 days of dexamethasone, whereas the remaining 2 infants received 7 days of dexamethasone. As expected, none of the infants with structural airway disease were successfully extubated with systemic steroid therapy. Exclusion of these infants from the analysis did not affect the results of our study.
Overall, 61% of infants treated with systemic steroids in our study responded with successful extubation. This finding is consistent with other studies that have reported successful extubation rates ranging from 47 to 76%.3, 13, 14, 20 This variability in clinical response to steroids was present regardless of whether infants were treated with high-dose or low-dose regimens of dexamethasone. We also reviewed each case individually to confirm that infants with failed extubations did not have other extrinsic reasons for failing extubation, such as infection or mechanical issues with nasal continuous positive airway pressure interface. The reason why some infants respond favorably to steroids while others do not is poorly understood. It is likely that a ‘one size fits all’ dosing strategy, although effective for most infants, may not work for some and may even be harmful for others.25 Future studies should be directed at the various factors that regulate variability in response to dexamethasone so that more individualized dosing that minimizes harm while maximizing benefit can be achieved.
A major limitation of our study was its relatively small sample size, which increases the likelihood of a type II error. In addition, its retrospective study design prevents us from determining the rationale for choosing a particular dexamethasone dosing schedule over another. Selection bias, such that infants deemed more critically ill were treated with the higher dose 10-day regimen, could not be ruled out, although the similar baseline clinical characteristics of the two groups at the start of steroid treatment make this less likely. Treatment decisions, including ventilator weaning and extubation, as well as reintubation and need for repeat course of steroids, were made by a diverse group of neonatologists with different management approaches. This lack of standardized approach could also have been a source of potential bias—although it does reflect clinical practice more accurately. Our study may also be biased toward the inclusion of sicker infants who were referred from other institutions due to their critical illness.
Despite the limitations of this study, our results provide further evidence for the use of low-dose dexamethasone in facilitating extubation of preterm infants with chronic lung disease. Until more information from randomized controlled trials can inform us of the optimal safe dexamethasone dose to use, it appears prudent to give the lowest possible dose for the shortest possible time whenever steroids are used in the prevention or treatment of BPD.
References
Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr 2005; 146 (6): 798–804.
Cummings JJ, D'Eugenio DB, Gross SJ . A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. N Engl J Med 1989; 320 (23): 1505–1510.
Ohlsson A, Calvert SA, Hosking M, Shennan AT . Randomized controlled trial of dexamethasone treatment in very-low-birth-weight infants with ventilator-dependent chronic lung disease. Acta Paediatr 1992; 81 (10): 751–756.
Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C . A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics 2002; 109 (2): 262–268.
Kothadia JM, O'Shea TM, Roberts D, Auringer ST, Weaver RG 3rd, Dillard RG . Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants. Pediatrics 1999; 104 (1 Pt 1): 22–27.
Barrington KJ . The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr 2001; 1: 1.
Halliday HL, Ehrenkranz RA, Doyle LW . Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev 2009; (1): CD001146.
Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed 2000; 83 (3): F177–F181.
O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 1999; 104 (1 Pt 1): 15–21.
Bhatt AJ, Feng Y, Wang J, Famuyide M, Hersey K . Dexamethasone induces apoptosis of progenitor cells in the subventricular zone and dentate gyrus of developing rat brain. J Neurosci Res 2013; 91 (9): 1191–1202.
Feng Y, Rhodes PG, Liu H, Bhatt AJ . Dexamethasone induces neurodegeneration but also up-regulates vascular endothelial growth factor A in neonatal rat brains. Neuroscience 2009; 158 (2): 823–832.
Irakam A, Miskolci V, Vancurova I, Davidson D . Dose-related inhibition of proinflammatory cytokine release from neutrophils of the newborn by dexamethasone, betamethasone, and hydrocortisone. Biol Neonate 2002; 82 (2): 89–95.
Doyle LW, Davis PG, Morley CJ, McPhee A, Carlin JB, Investigators DS . Low-dose dexamethasone facilitates extubation among chronically ventilator-dependent infants: a multicenter, international, randomized, controlled trial. Pediatrics 2006; 117 (1): 75–83.
Walther FJ, Findlay RD, Durand M . Adrenal suppression and extubation rate after moderately early low-dose dexamethasone therapy in very preterm infants. Early Hum Dev 2003; 74 (1): 37–45.
Watterberg KL American Academy of Pediatrics. Committee on F, Newborn. Policy statement—postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010; 126 (4): 800–808.
Bloomfield FH, Knight DB, Harding JE . Side effects of 2 different dexamethasone courses for preterm infants at risk of chronic lung disease: a randomized trial. J Pediatr 1998; 133 (3): 395–400.
Odd DE, Armstrong DL, Teele RL, Kuschel CA, Harding JE . A randomized trial of two dexamethasone regimens to reduce side-effects in infants treated for chronic lung disease of prematurity. J Paediatr Child Health 2004; 40 (5-6): 282–289.
Jones BO, Davies MW . Total steroid dose given to ventilated newborn infants with chronic lung disease. J Paediatr Child Health 2007; 43 (1-2): 40–43.
Malloy C, Hilal K, Rizvi Z, Weiss M, Muraskas J . A prospective, randomized, double-masked trial comparing low dose to conventional dose dexamethasone in neonatal chronic lung disease. Internet J Pediatr Neonatol 2005; 5: 1.
Rozycki HJ, Byron PR, Elliott GR, Carroll T, Gutcher GR . Randomized controlled trial of three different doses of aerosol beclomethasone versus systemic dexamethasone to promote extubation in ventilated premature infants. Pediatr Pulmonol 2003; 35 (5): 375–383.
Tanney K, Davis J, Halliday HL, Sweet DG . Extremely low-dose dexamethasone to facilitate extubation in mechanically ventilated preterm babies. Neonatology 2011; 100 (3): 285–289.
Yates HL, Newell SJ . Minidex: very low dose dexamethasone (0.05 mg/kg/day) in chronic lung disease. Arch Dis Child Fetal Neonatal Ed 2011; 96 (3): F190–F194.
Yates HL, Johnson K, Turner MA . The Minidex trial: does very low dose dexamethasone improve lung function in preterm infants? Infant 2016; 12 (2): 49–51.
Jobe AH . Postnatal corticosteroids for bronchopulmonary dysplasia. Clin Perinatol 2009; 36 (1): 177–188.
Wilkinson GR . Drug metabolism and variability among patients in drug response. N Engl J Med 2005; 352 (21): 2211–2221.
Acknowledgements
We would like to thank the Medical Writing Center at Children’s Mercy Kansas City for their assistance in proofreading and editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cuna, A., Govindarajan, S., Oschman, A. et al. A comparison of 7-day versus 10-day course of low-dose dexamethasone for chronically ventilated preterm infants. J Perinatol 37, 301–305 (2017). https://doi.org/10.1038/jp.2016.215
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2016.215
This article is cited by
-
Using quality improvement to implement consensus guidelines for postnatal steroid treatment of preterm infants with developing bronchopulmonary dysplasia
Journal of Perinatology (2021)
-
Effectiveness and safety of repeat dexamethasone for bronchopulmonary dysplasia
Journal of Perinatology (2021)
-
Efficacy of late postnatal dexamethasone on weaning from invasive mechanical ventilation in extreme premature infants
Journal of Perinatology (2021)