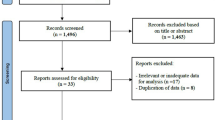
Abstract
Objective:
Bronchopulmonary dysplasia (BPD) is the most common cause of pulmonary morbidity in premature infants and is associated with life-long morbidities. Developing drugs for the prevention of BPD would improve public health. We sought to determine characteristics of favorable randomized controlled trials (RCTs) of drugs for BPD prevention.
Study Design:
We searched MEDLINE and EMBASE from 1992 to 2014 using the MeSH terms ‘BPD’ and ‘respiratory distress syndrome, newborn’. We included a Cochrane Library search to ensure inclusion of all available RCTs. We identified RCTs with BPD as a primary or secondary outcome and determined the definition of BPD used by the study. We determined whether a phase I or phase II study—to determine drug safety, efficacy or optimal dose—was performed before the RCT. Finally, we searched the Cochrane Library for meta-analyses for each drug and used the results of available meta-analyses to define a favorable versus unfavorable RCT.
Result:
We identified 2026 articles; 47 RCTs met our inclusion criteria encompassing 21 drugs; 5 of the drugs reduced the incidence of BPD. We found data from phase I or II studies for 16 of the drugs, but only 1 demonstrated a reduction of BPD.
Conclusion:
The majority of the drugs studied in RCTs failed to reduce the incidence of BPD. Performing early-phase studies before phase III trials might provide necessary information on drugs and drug doses capable of preventing BPD, thus informing the development of future RCTs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126: 443–456.
Baraldi E, Filippone M . Chronic lung disease after premature birth. N Engl J Med 2007; 357: 1946–1955.
Bhandari A, Panitch HB . Pulmonary outcomes in bronchopulmonary dysplasia. Semin Perinatol 2006; 30: 219–226.
Anderson PJ, Doyle LW . Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol 2006; 30: 227–232.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163: 1723–1729.
Jobe AH . The new bronchopulmonary dysplasia. Curr Opin Pediatr 2011; 23: 167–172.
Ambalavanan N, Walsh M, Bobashev G, Das A, Levine B, Carlo WA et al. Intercenter differences in bronchopulmonary dysplasia or death among very low birth weight infants. Pediatrics 2011; 127: e106–e116.
Baveja R, Christou H . Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 2006; 30: 209–218.
Strand M, Jobe AH . The multiple negative randomized controlled trials in perinatology—why? Semin Perinatol 2003; 27: 343–350.
Swartz MK . The PRISMA statement: a guideline for systematic reviews and meta-analyses. J Pediatr Health Care 2011; 25: 1–2.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004; 114: 1305–1311.
Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 1999; 340: 1962–1968.
Pearson E, Bose C, Snidow T, Ransom L, Young T, Bose G et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr 1992; 121: 420–427.
Kennedy KA, Stoll BJ, Ehrenkranz RA, Oh W, Wright LL, Stevenson DK et al. Vitamin A to prevent bronchopulmonary dysplasia in very-low-birth-weight infants: has the dose been too low? The NICHD Neonatal Research Network. Early Hum Dev 1997; 49: 19–31.
Darlow BA, Graham PJ . Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev (online) 2011; (10) CD000501.
Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006; 354: 2112–2121.
Picone S, Bedetta M, Paolillo P . Caffeine citrate: when and for how long. A literature review. J Matern Fetal Neonatal Med 2012; 25 (Suppl 3): 11–14.
Daily Med, US National Library of Medicine. Dexamethasone sodium phosphate injection. Available at: http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0277cc0a-2fd4-4605-a310-b613be84ee26 (last accessed 2 January 2014).
Anttila E, Peltoniemi O, Haumont D, Herting E, ter Horst H, Heinonen K et al. Early neonatal dexamethasone treatment for prevention of bronchopulmonary dysplasia. Randomised trial and meta-analysis evaluating the duration of dexamethasone therapy. Eur J Pediatr 2005; 164: 472–481.
Garland JS, Alex CP, Pauly TH, Whitehead VL, Brand J, Winston JF et al. A three-day course of dexamethasone therapy to prevent chronic lung disease in ventilated neonates: a randomized trial. Pediatrics 1999; 104 (1, Part 1): 91–99.
Lin YJ, Yeh TF, Hsieh WS, Chi YC, Lin HC, Lin CH . Prevention of chronic lung disease in preterm infants by early postnatal dexamethasone therapy. Pediatr Pulmonol 1999; 27: 21–26.
Rastogi A, Akintorin SM, Bez ML, Morales P, Pildes RS . A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant-treated infants. Pediatrics 1996; 98 (2, Part 1): 204–210.
Romagnoli C, Zecca E, Vento G, De Carolis MP, Papacci P Tortorolo G . Early postnatal dexamethasone for the prevention of chronic lung disease in high-risk preterm infants. Intens Care Med 1999; 25: 717–721.
Shinwell ES, Karplus M, Zmora E, Reich D, Rothschild A, Blazer S et al. Failure of early postnatal dexamethasone to prevent chronic lung disease in infants with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed 1996; 74: F33–F37.
Sinkin RA, Dweck HS, Horgan MJ, Gallaher KJ, Cox C, Maniscalco WM et al. Early dexamethasone-attempting to prevent chronic lung disease. Pediatrics 2000; 105 (3, Part 1): 542–548.
Tapia JL, Ramírez R, Cifuentes J, Fabres J, Hübner ME, Bancalari A et al. The effect of early dexamethasone administration on bronchopulmonary dysplasia in preterm infants with respiratory distress syndrome. J Pediatr 1998; 132: 48–52.
Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY et al. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 1997; 100: E3.
Kothadia JM, O'Shea TM, Roberts D, Auringer ST, Weaver I, Dillard RG . Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants. Pediatrics 1999; 104: 22–27.
Sanders RJ, Cox C, Phelps DL, Sinkin RA . Two doses of early intravenous dexamethasone for the prevention of bronchopulmonary dysplasia in babies with respiratory distress syndrome. Pediatr Res 1994; 36 (1, Part 1): 122–128.
Halliday HL, Ehrenkranz RA, Doyle LW . Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database Syst Rev (online) 2009; (1) ) CD001145.
Halliday HL, Ehrenkranz RA, Doyle LW . Early (<8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev (online) 2010; (1) CD001146.
Halliday HL, Ehrenkranz RA, Doyle LW . Moderately early (7–14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database Syst Rev (online) 2003; (1) CD001144.
Watterberg KL . Policy statement—postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010; 126: 800–808.
Toce SS, Farrell PM, Leavitt LA, Samuels DP, Edwards DK . Clinical and roentgenographic scoring systems for assessing bronchopulmonary dysplasia. Am J Dis Child 1984; 138: 581–585.
Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M . Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med 1992; 326: 1233–1239.
Phelps DL, Ward RM, Williams RL, Watterberg KL, Laptook AR, Wrage LA et al. Pharmacokinetics and safety of a single intravenous dose of myo-inositol in preterm infants of 23–29 wk. Pediatr Res 2013; 74: 721–729.
Ozdemir R, Erdeve O, Dizdar EA, Oguz SS, Uras N, Saygan S et al. Clarithromycin in preventing bronchopulmonary dysplasia in urea plasma urealyticum-positive preterm infants. Pediatrics 2011; 128: e1496–e1501.
Stevenson D, Walther F, Long W, Sell M, Pauly T, Gong A et al. Controlled trial of a single dose of synthetic surfactant at birth in premature infants weighing 500 to 699 grams. The American Exosurf Neonatal Study Group I. J Pediatr 1992; 120 (2, Part 2): S3–12.
McMillan D, Chernick V, Finer N, Schiff D, Bard H, Watts J et al. Effects of two rescue doses of synthetic surfactant in 344 infants with respiratory distress syndrome weighing 750 to 1249 grams: a double-blind, placebo-controlled multicenter Canadian trial. Canadian Exosurf Neonatal Study Group. J Pediatr 1995; 126 (5, Part 2): S90–S98.
Konishi M, Fujiwara T, Chida S, Maeta H, Shimada S, Kasai T et al. A prospective, randomized trial of early versus late administration of a single dose of surfactant-TA. Early Hum Dev 1992; 29 (1–3): 275–282.
Gortner L, Bartmann P, Pohlandt F, Bernsau U, Porz F, Hellwege HH et al. Early treatment of respiratory distress syndrome with bovine surfactant in very preterm infants: a multicenter controlled clinical trial. Pediatr Pulmonol 1992; 14: 4–9.
Soll RF . Synthetic surfactant for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev (online) 2000; (2) CD001149.
Soll R, Ozek E . Prophylactic protein free synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev (online) 2010; (1) CD001079.
Seger N, Soll R . Animal derived surfactant extract for treatment of respiratory distress syndrome. Cochrane Database Syst Rev (online) 2009; (2) CD007836.
Barrington KJ, Finer NN . Inhaled nitric oxide for respiratory failure in preterm infants. Cochrane Database Syst Rev (online) 2007; (3)CD000509.
Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A et al. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr 2005; 146: 318–323.
Watterberg KL, Gerdes JS, Gifford KL, Lin HM . Prophylaxis against early adrenal insufficiency to prevent chronic lung disease in premature infants. Pediatrics 1999; 104: 1258–1263.
Ahola T, Fellman V, Laaksonen R, Laitila J, Lapatto R, Neuvonen PJ et al. Pharmacokinetics of intravenous N-acetylcysteine in pre-term new-born infants. Eur J Clin Pharmacol 1999; 55: 645–650.
Viscardi RM, Othman AA, Hassan HE, Eddington ND, Abebe E, Terrin ML et al. Azithromycin to prevent bronchopulmonary dysplasia in ureaplasma-infected preterm infants: pharmacokinetics, safety, microbial response, and clinical outcomes with a 20-milligram-per-kilogram single intravenous dose. Antimicrob Agents Chemother 2013; 57: 2127–2133.
Hassan HE, Othman AA, Eddington ND, Duffy L, Xiao L, Waites KB et al. Pharmacokinetics, safety, and biologic effects of azithromycin in extremely preterm infants at risk for ureaplasma colonization and bronchopulmonary dysplasia. J Clin Pharmacol 2011; 51: 1264–1275.
Ballard HO, Anstead MI, Shook LA . Azithromycin in the extremely low birth weight infant for the prevention of bronchopulmonary dysplasia: a pilot study. Respir Res 2007; 8: 41.
Ballard HO, Shook LA, Bernard P, Anstead MI, Kuhn R, Whitehead V et al. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatr Pulmonol 2011; 46: 111–118.
Trotter A, Maier L, Grill HJ, Kohn T, Heckmann M, Pohlandt F . Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J Clin Endocrinol Metab 1999; 84: 4531–4535.
Denjean A, Paris-Llado J, Zupan V, Debillon T, Kieffer F, Magny JF et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: a randomised double-blind study. Eur J Pediatr 1998; 157: 926–931.
Rosenfeld WN, Davis JM, Parton L, Richter SE, Price A, Flaster E et al. Safety and pharmacokinetics of recombinant human superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics 1996; 97 (6, Part 1): 811–817.
Cole CH, Colton T, Shah BL, Abbasi S, MacKinnon BL, Demissie S et al. Early inhaled glucocorticoid therapy to prevent bronchopulmonary dysplasia. N Engl J Med 1999; 340: 1005–1010.
Viscardi RM, Hasday JD, Gumpper KF, Taciak V, Campbell AB, Palmer TW . Cromolyn sodium prophylaxis inhibits pulmonary proinflammatory cytokines in infants at high risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med 1997; 156: 1523–1529.
Fok TF, Lam K, Dolovich M, Ng PC, Wong W, Cheung KL et al. Randomised controlled study of early use of inhaled corticosteroid in preterm infants with respiratory distress syndrome. Arch Dis Child Fetal Neonatal Ed 1999; 80: F203–F208.
Terrin G, Canani RB, Passariello A, Messina F, Conti MG, Caoci S et al. Zinc supplementation reduces morbidity and mortality in very-lowbirth-weight preterm neonates: a hospital-based randomized, placebo-controlled trial in an industrialized country. Am J Clin Nutrition 2013; 98: 1468–1474.
Ahola T, Lapatto R, Raivio KO, Selander B, Stigson L, Jonsson B et al. N-acetylcysteine does not prevent bronchopulmonary dysplasia in immature infants: a randomized controlled trial. J Pediatr 2003; 143: 713–719.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116: 1353–1360.
Food and Drug Administration (FDA). FDA Guidance for Industry: Drug Approval Process. 2000. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/default.htm (last accessed 2 January 2014).
Institute of Medicine. Safe and Effective Medicines for Children: Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. National Academy of Sciences: Washington, DC, USA, 2012.
Dunne J, Rodriguez WJ, Murphy MD, Beasley BN, Burckart GJ, Filie JD et al. Extrapolation of adult data and other data in pediatric drug-development programs. Pediatrics 2011; 128: e1242–e1249.
Food and Drug Administration (FDA). FDA Guidance for Industry: Clinical Investigation of Medicinal Products in the Pediatric Population. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073143.pdf (last ccessed 2 January 2014).
Food and Drug Administration (FDA). FDA Guidance for Industry: How to Comply with the Pediatric Research Equity Act. Available at: http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM077855.pdf (last accessed 2 January 2014).
Laughon MM, Benjamin DK Jr, Capparelli EV, Kearns GL, Berezny K, Paul IM et al. Innovative clinical trial design for pediatric therapeutics. Expert Rev Clin Pharmacol 2011; 4: 643–652.
Shamliyan T, Kane RL . Clinical research involving children: registration, completeness, and publication. Pediatrics 2012; 129: e1291–e1300.
Acknowledgements
We thank and acknowledge Kathleen McGraw for her expertise in assisting in the development of the literature search. Dr Cohen-Wolkowiez receives support for research from the National Institutes of Health (NIH) (1K23HD064814), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization Thrasher Research Fund (http://www.thrasherresearch.org) and from industry for drug development in adults and children (http://www.dcri.duke.edu/research/coi.jsp). Dr Smith receives salary support for research from the NIH, the US Department of Health and Human Services and the National Center for Advancing Translational Sciences of the NIH (DHHS-1R18AE000028-01, HHSN267200700051C, HHSN275201000003I and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr Laughon receives support from the US government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin under the Best Pharmaceuticals for Children Act) and from NICHD (1K23HL092225-01); he also receives support from Astellas, Pfizer, Abbvie and Discovery Laboratories for his work on data safety monitoring boards and consulting. This work was supported by a grant from the Doris Duke Charitable Foundation to UNC–Chapel Hill School of Medicine to fund clinical research fellow Kristyn S Beam. The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant award numbers UL1TR000083 and UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Beam, K., Aliaga, S., Ahlfeld, S. et al. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol 34, 705–710 (2014). https://doi.org/10.1038/jp.2014.126
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2014.126
This article is cited by
-
A modified lung ultrasound score to evaluate short-term clinical outcomes of bronchopulmonary dysplasia
BMC Pulmonary Medicine (2022)
-
Safety of sildenafil in extremely premature infants: a phase I trial
Journal of Perinatology (2022)
-
Low flow nasal cannula requirement among preterm infants: predictors and description of clinical course
Journal of Perinatology (2022)
-
Specificity of International Classification of Diseases codes for bronchopulmonary dysplasia: an investigation using electronic health record data and a large insurance database
Journal of Perinatology (2021)
-
Definitions of bronchopulmonary dysplasia and long-term outcomes of extremely preterm infants in Korean Neonatal Network
Scientific Reports (2021)