Abstract
Objective:
Assess the impact of intercurrent respiratory infections in infants <29 weeks gestational age (GA).
Study design:
A retrospective cohort study of 111 infants born <29 weeks GA, controlling for bronchopulmonary dysplasia (BPD) severity and assessing pulmonary health over the first year of life through oxygen, diuretic and inhaled steroid use.
Result:
Regression analysis showed viral infections increased oxygen use (odds ratio (OR) of 15.5 (confidence interval (CI)=3.4, 71.3)). The trend test showed increasing numbers of viral infections were associated with increased oxygen (OR (95% CI)=6.4 (2.3 to 17.4), P=0.0003), diuretic (OR (95% CI)=2.4 (1.1to 5.2), P=0.02) and inhaled steroid use (OR (95% CI)=2.2 (1.003 to 5.2), P=0.049), whereas bacterial infections were not.
Conclusion:
Viral infections caused more long-term pulmonary morbidity/mortality than bacterial infections on premature lung health, even when controlling for BPD.
Similar content being viewed by others
Introduction
One in eight infants is born prematurely in the United States annually, costing ∼$26.2 billion dollars a year ($51 600 per infant) on their medical care.1, 2 Infants born in the United States <28 weeks gestational age (GA) now have a 90% survival rate3 with chronic respiratory morbidity as the most common and costly adverse outcome for these infants.4, 5, 6
Premature infants’ altered lung development places them at risk for bronchopulmonary dysplasia (BPD) in the first year of life. BPD is now characterized by less inflammation and fibrosis of the airways but with structural abnormalities.6, 7 A total of 77% of infants born <30 weeks GA develop BPD.4 Some of these infants manifest early mild respiratory problems that deteriorate over the first year of life.6 These pulmonary abnormalities are not always associated with the infants’ severity of BPD around 36 weeks GA or respiratory support in the Neonatal Intensive Care Unit (NICU).8
Intercurrent respiratory infections in this group have been associated with increased morbidity and premature infants have a 73% chance of readmission to the hospital for respiratory infections in the first 2 years of life.9 Respiratory syncyntial virus is the most common cause for readmission;10 however, rhinovirus, the most common viral pathogen in childhood, can also cause severe respiratory infections in premature infants.11, 12, 13 The primary structural and functional problems in BPD, in combination with intercurrent respiratory infections, may be creating the conditions for morbidity and death among infants who appeared to be developing successfully. Infants with milder BPD may be at risk for increased hospitalization over time from respiratory complications. The exact cause of this increased morbidity is unclear.6
The objective of this study was to evaluate the additional impact of intercurrent bacterial and/or viral respiratory infections on a cohort of extremely premature infants’ pulmonary health over the first year of life past NICU discharge regardless of re-hospitalization status.
Methods
Data repository
The data repository of the Center for Infant Pulmonary Disorders is located at Children’s Mercy Hospitals and Clinics (CMH). It provided de-identified data for this study according to the process approved by the Institutional Review Board at CMH. Infants hospitalized at CMH were included in the data repository if they were born <29 weeks GA, born from 1 January 2008 to 31 December 2010, born on-site or transferred to CMH within 24 h of life and no diagnosis of major anomalies. The data repository collects information on the cohort from birth through 5 years of life.
Determination of BPD
Classification of BPD was performed at 36 weeks GA and used the criteria outlined by Ehrenkranz et al. in 2005 for infants born <32 weeks GA.5 An infant was defined as no BPD if he/she was maintaining SpO2 >90% breathing ambient air at 36 weeks or upon discharge from the NICU (whichever came first) and if the child was treated with supplemental oxygen for <28 days. A diagnosis of BPD was given if the patient required supplemental oxygen to keep their SpO2 >90% for at least 28 days. Infants were classified as: mild BPD, included patients who requiring supplemental oxygen to keep the SpO2 >90% for >28 days but were on room air by discharge or at 36 weeks gestation; moderate BPD, if they were requiring <30% supplemental oxygen at 36 weeks GA to maintain their SpO2 >90% or upon discharge; or severe BPD, if they were requiring 30% or more supplemental oxygen at 36 weeks GA/discharge to maintain their SpO2 >90% or were on positive pressure ventilation at 36 weeks. The confounding variable of BPD severity was controlled by stratifying our cohort into three groups according to their BPD classification: none/mild, moderate and severe.
Definition of infection
CMH follows the National Health Safety Network criteria for diagnosing and reporting infections. A bacterial airway infection was diagnosed in the NICU if all of the following criteria were met: sustained (>1 day) deterioration in physical exam or respiratory status, laboratory/radiographic evidence for infection including elevated white blood cell count with a shift and elevated C reactive protein, a bacterial isolate consistent with a respiratory pathogen obtained from a tracheal aspirate, sputum sample or nasal aspirate, and initiation of >7 days of antibiotic therapy. Patients who were discharged from the hospital were considered to have a bacterial infection if they were given the diagnosis of a bacterial pneumonia/bronchitis from an emergency room (ER) physician, pediatrician or hospitalist (with or without supporting laboratory data) and prescribed antibiotics for >7 days.
A viral infection was identified in the NICU when the patient was clinically symptomatic (which included: increased oxygen requirement, tachypnea, increased nasal/oral secretions, increased apnea/bradycardia spells and changes on chest radiograph) and had a virus isolated from a viral PCR/culture. The viral PCR used in-house at CMH during this time was the Luminex RVP (Luminex, Austin, TX, USA), which tested for Influenza A, Influenza A subtype H1, Influenza A subtype H3, Influenza B, respiratory syncyntial virus subtype A, respiratory syncyntial virus subtype B, Parainfluenza 1–3, Human Metapneumovirus, Rhinovirus/Enterovirus and Adenovirus. Routine screening for viral pathogens did not occur in the NICU. Patients who were discharged from the NICU were considered to have a viral infection if they were given the diagnosis of a viral respiratory infection from an ER physician, pediatrician or hospitalist (with or without supporting laboratory data). The patient charts were analyzed up to 1 year post NICU discharge for evidence of intercurrent respiratory infections by trained data abstractors. Medical record reviews included evaluation of the discharge summary, 6 and 12 months follow-up visits in the special care clinic, ER notes and hospital discharge notes.
Study population
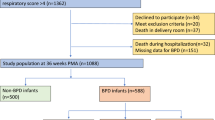
We included all infants enrolled in the data repository between 01 January 2008 and 31 December 2010 who were <29 weeks GA at birth and admitted to CMH within 24 h of birth. We excluded infants who died <36 weeks corrected GA, were >29 weeks GA at birth or had significant congenital heart disease or structural airway abnormalities. Of 184 liveborn infants, 157 infants were initially identified in the data repository for consideration in this study. Fifteen infants died <36 weeks corrected GA, leaving a total of 142 infants who met criteria for entry into the study. Only one patient had a tracheostomy and none were ventilator dependent. The tracheostomy/ventilator-dependent patients during this time frame had been transferred from outside centers for a second opinion several weeks after delivery.
Study design
A retrospective (historical) cohort design was used to evaluate the impact of intercurrent respiratory infections on lung health in extremely premature infants up to 1 year of age. The primary outcome variables of oxygen requirement, diuretic and inhaled steroid use at 12 months of age were selected based on their common use in infants with pulmonary sequelae from BPD in an outpatient setting.
Data analysis
Statistical analysis used the SAS programming version 9.2 software package (SAS Institute, Cary, NC, USA). We first performed bivariate analysis between risk factors (BPD, viral infection and bacterial infection) and outcome variables (oxygen use, diuretics use and inhaled steroid) using either the Cochran–Mantel Haenszel χ2 test and/or Fisher’s exact test. The outcome variables of interest were determined through medical record reviews of the discharge summary, 6 and 12 months follow-up visits in the Special Care Clinic, ER notes and hospital discharge notes. A stratified analysis was then used to assess the impact of intercurrent bacterial and viral respiratory infections on the entire cohort and on each individual BPD group. The Cochran–Armitage trend test was used to evaluate the impact of multiple infections on the three outcome variables.
A secondary outcome variable of death after NICU discharge was also evaluated. We developed a multivariable model using logistic regression with backward model selection. In the multivariate analysis, we considered GA, gender, severity of BPD, viral infection, bacterial infection, viral/bacterial interaction, BPD/viral interaction, BPD/bacterial interaction and birthweight on their association with the outcome variables. To keep model parsimony, we started with a full model with all predictive variables as mentioned above and then used backward elimination to remove nonsignificant risk factors (P>0.05). After backward elimination, only significant risk factors remained in the logistic regression model. Odds ratio (OR) and 95% confidence interval (CI) of OR were reported. To keep model parsimony, nonsignificant factors are removed from the multivariate models. Ultimately, the final model included the following variables: GA, severity of BPD, viral infection and bacterial infection. Statistical significance was claimed with P<0.05.
Results
Basic demographic information on gender, GA and birthweight was collected on each infant in the final cohort (Table 1). Of the initial 157 infants, 15 died <36 weeks corrected GA (Figure 1). This left a sample size of 142 patients that underwent BPD classification.5 These infants were then separated into three groups for our study, which included no/mild BPD (66/142), moderate BPD (45/142) and severe BPD (31/142). Of the 142 children, 6 patients died and 25 were lost to follow-up (LFU), including 14 patients LFU (20%: 6 female/8 male with average GA 27 weeks) and 1 death in the no/mild BPD group, 7 patients LFU (15%: 4 female/3 male with average GA 26 weeks) and 3 deaths in the moderate BPD group and 4 patients LFU (13%: 2 female/2 male with average GA 26 weeks) and 2 deaths in the severe BPD group by the 12-month follow-up period. They were therefore excluded in the final sample (N=111). Complete data were available on 51 patients in the no/mild BPD group, 35 patients in the moderate BPD group and 25 patients in the severe BPD group. Of the 35 patients in the mild/no BPD group there were 17 bacterial infections and 5 viral infections identified; 2 patients had overlapping infections identified. Of the 35 patients in the moderate BPD group there were 15 bacterial infections and 7 viral infections identified; 2 patients had overlapping infections identified. Of the 25 patients in the severe BPD group there were 17 bacterial infections and 6 viral infections identified; 4 patients had overlapping infections identified. Increasing BPD severity was associated with increasing frequency of supplemental oxygen use, diuretic use and inhaled steroid use among patients at anytime up to 1 year of age corrected with no/mild, moderate and severe BPD, respectively (P<0.0003, P<0.0001 and P<0.0001). Oxygen use was 6%, 11% and 40%, inhaled steroid use was 3%, 16% and 39%, and diuretic use was 14%, 44% and 71%, respectively.
To evaluate the impact of intercurrent respiratory tract infections on three primary outcome variables (oxygen use, inhaled steroid use and diuretic use at 12 months) in each BPD group, we then used a stratified bivariate analysis using the Cochran–Mantel Haenszel χ2 modeling to assess the impact of both bacterial and viral infections on our outcome variables in each of the defined BPD groups. A logarithmic regression model showed that there was not significant interaction between the viral and bacterial infections (OR (95%)=2.1(0.1, 38.3), P=0.61).
Intercurrent bacterial and viral infections were associated with significantly higher oxygen use when all BPD groups were combined with a significantly greater impact seen in the viral group (8% vs 24%, P=0.02 and 9% vs 47%, P<0.0002, respectively; Table 2). Bacterial infections did not statistically influence the oxygen use in any of the three BPD sub-groups. Intercurrent viral infections did statistically increase oxygen use at 12 months post NICU discharge: 2% vs 40% O2 use in the no/mild BPD (P=0.02) and 26% vs 83% in the severe BPD group (P=0.02; Table 2).
The intercurrent bacterial and viral infections were associated with increased inhaled steroid use in the combined BPD groups, but not in any individual BPD groupings. Bacterial infections increased use from 9 to 22% (P=0.03) and viral infections increased use from 11 to 29% (P=0.01; Table 2).
The intercurrent respiratory infections were associated with increased diuretic use in the viral group only. The viral impact on the combined BPD group analysis was 32% vs 57% (P=0.02). When the BPD subgroups were broken apart, the no/mild BPD group continued to show a significant increase in diuretic use (10% vs 50%, P=0.03; Table 2).
The data were then analyzed, using the Cochran–Armitage trend test, to see if an increasing number of viral or bacterial infections was associated with a significant impact on the three primary outcome variables. We first looked at the association of increasing number of viral infections on oxygen use. An increasing number of viral infections was associated with a significant impact on oxygen use (OR (95% CI)=6.4 (2.3 to 17.4), P=0.0003): at baseline 9% of the cohort required oxygen, which increased with increasing number of viral infections to 44% with one viral infection and 67% with two viral infections, diuretic use (OR (95% CI)=2.4 (1.1 to 5.2), P=0.02): at baseline 32% of the cohort required diuretics, which increased with increasing number of viral infections to 53% with one viral infection and 75% with two viral infections, and inhaled steroid use (OR (95% CI)=2.2 (1.003 to 5.2), P=0.049): at baseline 12% of the cohort required inhaled steroids, which increased with increasing number of viral infections to 32% with one viral infection and 25% with two viral infections. With increasing bacterial infections, we saw an increase in oxygen use from 8% at baseline to 24% with one infection, 29% with two infections (P=0.05), an increase in diuretics use from 31% at baseline to 41% with one infection, 50% with two infections (P=0.07), and an increase in oxygen use from 9% at baseline to 22% with one infection, 25% with two infections (P=0.07), but they are not statistically significant.
Three separate logistic regression models were created, one for each of our three primary outcome variables. We forced the following variables into each model: GA, BPD class, viral infection and bacterial infection). We originally included the variables gender and viral*bacterial variables but these were excluded after backward stepwise regression. Viral infections were shown to increase oxygen use (OR (95% CI)=15.6 (3.4, 71.3), P=0.0004; Table 3). The model had 89.3% prediction accuracy. There was no significant interaction between viral and bacterial infections (OR (95% CI)=2.1 (0.1, 38.3), P=0.61).
Finally, the secondary outcome variable of death after NICU discharge was evaluated using bivariate analysis. Seven infants were identified (5% of the cohort) who died after NICU discharge. Six out of seven infants had recent or ongoing viral infections at the time of their death (P<0.0001). One had mild BPD, three had moderate BPD and two had severe BPD. These deaths do not appear to correlate with the 36-week BPD severity assessment. Three out of six infants had a history of adrenal insufficiency during their NICU stay and four out of six infants had a history of transient pulmonary hypertension in the NICU; however, the sample size for these observations were too small to draw any meaningful conclusions.
Discussion
This study evaluated the impact of both bacterial and viral infections on the same cohort of extremely premature infants over their first year of life while controlling for BPD severity. Previous literature has shown this population is at increased risk of re-hospitalization from pulmonary sequelae9 and viral infections could be severe in this population11, 12, 13 with one study finding it was the primary cause of readmission.10 However, no study compared both bacterial and viral impact on this population simultaneously or with controlling for BPD severity. We demonstrated that viral infections had a significantly greater impact compared with bacterial infections by evaluating commonly used therapies for chronic lung disease of infancy in the outpatient setting: oxygen use, diuretic use and inhaled steroid use. Increasing number of viral infections also had a significant impact on premature infant lung morbidity/mortality, whereas increasing number of bacterial infections did not.
To understand why viral infections appear to affect BPD lung physiology differently than bacterial infections it is important to infant's how BPD lung structure differs from a full-term infant's lung structure. BPD lung structure consists of a simplified alveolar architecture7, 14 with less alveolar area for ventilation as well as a decreased intravascular pulmonary network that affects the perfusion to the lung.15, 16, 17 In the short term, this leads to less baseline pulmonary reserve, which is further compromised by infection-mediated inflammation.
Viral infections have been shown to remain in lung tissue for over a month after the acute infection has resolved, causing prolonged abnormal inflammatory responses.18, 19 Viral infections have also been linked to the development of airway hyper reactivity and asthma in later life,20 which further demonstrates the long-lasting impact of viral infections on the pulmonary physiology. As viruses persist in the lung longer than bacteria, it is not surprising that our cohort had worse pulmonary outcomes at 1 year with a history of viral as opposed to bacterial intercurrent lung infections. Recent animal studies have indicated that supplemental oxygen increases an animal’s sensitivity to influenza infection through altered inflammatory pathways,21 which adds biologic plausibility to our clinical findings linking greater lung morbidity in premature infants at a year of age who have had intercurrent viral infections.
There were several limitations in our study design that must be considered when evaluating these results. All of these infants were from one center and our findings may not be generalizable to the population at large; however, comparison with one national database did show similar outcomes for BPD severity.22 Also, obtaining long-term data on extremely preterm infants can be challenging, especially for infants who are born >160 km from the admitting NICU center. Therefore, some infants were LFU after hospital discharge. Some of these infants could have moved out of state and died in another center or have significant pulmonary morbidity that was being managed at another location. Another limitation was our definition of infections. Although the patients were in the NICU, we had better control over management; once the patients were in the community, the type of work-up they obtained varied and did not always include the same supporting laboratory/radiographic studies. We identified one no/mild BPD, one moderate BPD and three severe BPD cases that relied on documented radiographic, laboratory and clinical exam findings to diagnose the viral upper respiratory tract infection but no actual virus was isolated on viral studies. Two no/mild, three moderate and one severe BPD case did not have confirmation on how the viral upper respiratory tract infection was diagnosed. During this time period, the extended viral PCR panel (Biofire FilmArray RP), which now includes an extended number of viral pathogens including Coronavirus 229E, Coronavirus HKU1, Coronavirus NL63, Coronavirus OC43, Influenza A subtype H1-2009, Parainfluenza virus 4, Bordetella pertussis, Chlamydophila pneumonia and Mycoplasma pneumoniae, was not commonly being used in our center or the community. Once discharged from the NICU, the infants were not evaluated on a daily basis by a physician and it is likely that minor infections, both bacterial/viral, went unnoticed by caregivers. Also, our center followed the American Academy of Pediatrics recommendation for Synagis administration. It was given 48 to 72 h before discharge between the months of November and March. Out of 157 patients, 51 were discharged within this timeframe and all received their initial Synagis dose in-house; however, subsequent doses were provided to their local pediatricians so compliance data are not available for this cohort. Potentially, a future prospective study would be better able to control for some of these limitations; however, even future prospective studies could have difficulties because currently the outcome variables used (diuretic use and inhaled steroid use) do not have nationally agreed upon routine use. Locally, we were consistent with our initiation of therapy and weaning protocols; however, this will not be the case if data from multi-center national databases are used, this further highlights the need for ongoing research into this area of clinical management for this population.
There were several strengths in our study design. We evaluated infants born recently (2008 to 2010) and had been hospitalized within the same hospital within 24 h of birth. This study design minimized the variability of ventilatory strategies used on this cohort as they were managed by the same group of physicians incorporating the goal of quick transition from invasive to noninvasive ventilatory support.22 As our cohort was born recently and spans only 3 years, the impact of changing technology and medical therapies over time was minimized. Our study also required access to medical records for this cohort past hospital discharge with special interest being placed on ER, hospital and primary care provider records. As this center is the only tertiary referral center for children with complex illness in this geographic region, we hospitalize over 86% of pediatric cases (unpublished CMH demographic data) and closer to 100% of high-risk cases. A multispecialty clinic follows the NICU graduates closely over the first few years of life with close coordination/communication with the outside pediatrician. This makes it less likely that an episode of illness requiring health-care utilization was missed. The NICU clinic sees the patients every 1 to 3 months for at least a year post NICU discharge and the CMH-DR has permission to collect information on participants up to 5 years post NICU discharge. Only 13% (19/142) of the cohort were LFU and 78% (111/142) of participants were successfully followed through their first year of life (82% if deaths were excluded (111/136)).
There is variability between discharge management for this patient population. Current post hospital BPD management focuses mainly on nutrition, growth and neurological outcome. Several strategies on home oxygen therapy and weaning protocols have been proposed;22, 23, 24, 25 however, there still remains a wide variability in practice in the pediatric providers.26 Even the efficacy of reducing health-care expenditures in this patient population with outpatient pulmonary follow-up is unclear. However, recent literature has shown that pulmonary follow-up keeps the ER visits and hospitalization rates between mild and severe BPD patients stable27 when normally more severe BPD patients have increased sequelae. Previous recommendations for evaluation of sleep disordered breathing in this population was focused on infants with severe BPD; however, recent studies have shown that daytime oxygen saturations and respiratory rates did not predict instability seen on sleep studies.28 Recent studies performing serial infant pulmonary function tests did not demonstrate any ‘catch up’ lung growth in the BPD infants in the first year of life.29, 30 Studies in teenagers/adults with a history of BPD showed persistent abnormalities in lung function as seen by increased air trapping, increased residual volume, increased incidence of airway hyper reactivity and a faster decline in lung function in adulthood compared with controls.31, 32, 33 Our study suggests that a wider range of extremely premature infants, even those with no/mild BPD, may be at risk of significant pulmonary sequelae from intercurrent infections based on this increased use of oxygen over the first year of life.
References
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379 (9832): 2162–2172.
Moken NH . Preterm birth: new data on a global health priority. Lancet 2012; 379 (9832): 2128–2130.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126 (3): 443–456.
Moster D, Lie RT, Markestad T . Long-term medical and social consequences of preterm birth. N Engl J Med 2008; 359 (3): 262–273.
Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116 (6): 1353–1360.
Greenough A . Long term respiratory outcomes of very premature birth (<32 weeks). Semin Fetal Neonatal Med 2012; 17 (2): 73–76.
Jobe AH, Bancalari E . Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163 (7): 1723–1729.
Anand D, Stevenson CJ, West CR, Pharoah PO . Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child 2003; 88 (2): 135–138.
Greenough A, Cox S, Alexander J, Lenney W, Turnbull F, Burgess S et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child 2001; 85 (6): 463–468.
Drysdale SB, Wilson T, Alcazar M, Broughton S, Zuckerman M, Smith M et al. Lung function prior to viral lower respiratory tract infections in prematurely born infants. Thorax 2011; 66 (6): 468–473.
Bennett NJ, Tabarani CM, Bartholoma NM, Wang D, Huang D, Riddell SW et al. Unrecognized viral respiratory tract infections in premature infants during their birth hospitalization: a prospective surveillance study in two neonatal intensive care units. J Pediatr 2012; 161 (5): 814–818.
Linder JE, Kraft DC, Mohamed Y, Lu Z, Heil L, Tollefson S et al. Human rhinovirus C: Age, season, and lower respiratory illness over the past 3 decades. J Allergy Clin Immunol 2013; 131 (1): 69–77 e1-6.
Regamey N, Kaiser L . Rhinovirus infections in infants: is respiratory syncytial virus ready for the challenge? Eur Respir J 2008; 32 (2): 249–251.
Reyburn B, Martin RJ, Prakash YS, MacFarlane PM . Mechanisms of injury to the preterm lung and airway: implications for long-term pulmonary outcome. Neonatology 2012; 101 (4): 345–352.
Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR et al. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005; 289 (4): L529–L535.
Balinotti JE, Chakr VC, Tiller C, Kimmel R, Coates C, Kisling J et al. Growth of lung parenchyma in infants and toddlers with chronic lung disease of infancy. Am J Respir Crit Care Med 2010; 181 (10): 1093–1097.
Mourani PM, Ivy DD, Gao D, Abman SH . Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004; 170 (9): 1006–1013.
Hegele RG, Hayashi S, Bramley AM, Hogg JC . Persistence of respiratory syncytial virus genome and protein after acute bronchiolitis in guinea pigs. Chest 1994; 105 (6): 1848–1854.
Estripeaut D, Torres JP, Somers CS, Tagliabue C, Khokhar S, Bhoj VG et al. Respiratory syncytial virus persistence in the lungs correlates with airway hyperreactivity in the mouse model. J Infect Dis 2008; 198 (10): 1435–1443.
Bacharier LB, Cohen R, Schweiger T, Yin-Declue H, Christie C, Zheng J et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2012; 130 (1): 91–100 e3.
O'Reilly MA, Yee M, Buczynski BW, Vitiello PF, Keng PC, Welle SL et al. Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear1. Am J Pathol 2012; 181 (2): 441–451.
Truog WE, Nyp MF, Taylor J, Gratny LL, Escobar H, Manimtim WM et al. Infants born at <29 weeks: pulmonary outcomes from a hybrid perinatal system. J Perinatol 2013 e-pub ahead of print; doi:10.1038/JP.2013.125.
Abman SH, Groothius JR . Pathophysiology and treatment of bronchopulmoanry dysplasia. Current issues. Pediatr Clin North Am 1994; 41 (2): 227–315.
Ellsbury DL, Acarregui MJ, McGuinness GA, Eastman DL, Klein JM . Controversy surrounding the use of home oxygen for premature infants with bronchopulmonary dysplasia. J Perinatol 2004; 24 (1): 36–40.
Groothuis JR, Makari D . Definition and outpatient management of the very low-birth-weight infant with bronchopulmonary dysplasia. Adv Ther 2012; 29 (4): 297–311.
Palm K, Simoneau T, Sawicki G, Rhein L . Assessment of current strategies for weaning premature infants from supplemental oxygen in the outpatient setting. Adv Neonatal Care 2011; 11 (5): 349–356.
Rhein LM, Konnikova L, McGeachey A, Pruchniewski M, Smith VC . The role of pulmonary follow-up in reducing health care utilization in infants with bronchopulmonary dysplasia. Clin Pediatr (Phila) 2012; 51 (7): 645–650.
McGrath-Morrow SA, Ryan T, McGinley BM, Okelo SO, Sterni LM, Collaco JM . Polysomnography in preterm infants and children with chronic lung disease. Pediatr Pulmonol 2012; 47 (2): 172–179.
Schmalisch G, Wilitzki S, Roehr CC, Proquitte H, Buhrer C . Development of lung function in very low birth weight infants with or without bronchopulmonary dysplasia: longitudinal assessment during the first 15 months of corrected age. BMC Pediatr 2012; 12: 37.
Fakhoury KF, Sellers C, Smith EO, Rama JA, Fan LL . Serial measurements of lung function in a cohort of young children with bronchopulmonary dysplasia. Pediatrics 2010; 125 (6): e1441–e1447.
Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM . Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics 2006; 118 (1): 108–113.
Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ . Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 2006; 173 (8): 890–896.
Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Carlsen KH, Bakke P . Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr 2004; 93 (10): 1294–1300.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Taylor, J., Nyp, M., Norberg, M. et al. Impact of intercurrent respiratory infections on lung health in infants born <29 weeks with bronchopulmonary dysplasia. J Perinatol 34, 223–228 (2014). https://doi.org/10.1038/jp.2013.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jp.2013.152