Abstract
Disruption of nitric oxide pathway and endoplasmic reticulum (ER) stress had been observed in preeclampsia (PE). However, the correlation and overall detailed expression profiles of ER stress-related markers and endothelial nitric oxide synthase/inducible nitric oxide synthase (eNOS/iNOS) in patients with PE were poorly understood. In this study, placental protein expression of ER stress-related markers as well as eNOS/iNOS in normotensive control (n=32) and PE pregnancies (n=32) was examined by western blot. In addition, apoptosis was detected by terminal deoxynucleotidyl transferase-mediated nick-end labelling (TUNEL) staining in placentas. Compared with control, we found elevated ER stress response was agreeable with iNOS upregulation in placenta tissue of PE patients. Placental protein expression of ER stress-related markers, including GRP78, GRP94, p-PERK, eIF2a, p-eIF2a, XBP1, CHOP, Ire1, p-Ire1 and iNOS, was higher, and eNOS expression was lower in PE (P<0.05 for all); however, the expression of ATF6 and PERK was similar in the PE and control groups. Upregulation of CHOP and iNOS was consistent of apoptosis increasing indicated by TUNEL staining and caspase 4 expression upregulation in PE placenta. Our datas suggest that the exaggerated ER stress response and upregulated iNOS are probably associated with increased apoptosis in placenta of PE patients and may contribute to the pathophysiology of PE.
Similar content being viewed by others
Introduction
Preeclampsia (PE) is a pregnancy-induced hypertensive disorder associated with proteinuria that occurs after the 20th gestational week. This disorder affects 5–8% of pregnancies, and it is highly related with perinatal morbidity and mortality.1, 2 Although the aetiology of PE has not been entirely clarified, the most widely held view is that trophoblast injury including invasion malfunction and apoptosis are key mechanisms in PE pathogenicity.3, 4, 5
Nitric oxide (NO) is a potent vasodilator and is thought to have a major effect on gestational vasodilation.6 NO production is catalyzed by the conversion of L-arginine to NO by NO synthase (NOS), including neuronal NOS, endothelial NOS (eNOS) and inducible NOS (iNOS). It is reported that eNOS and iNOS are expressed mainly on syncytiotrophoblasts and endothelial cells in the placenta during pregnancy.7, 8 However, their expression and role on trophoblasts in PE patients remain uncertain.
Endoplasmic reticulum (ER) stress, provoked by stress and an imbalance between the load of unfolded proteins in the ER and the capacity of the ER, leads to the accumulation of unfolded or misfolded proteins in the ER.9 This accumulation triggers the activation of three well-known pathways: the double-stranded RNA-activated protein kinase-like ER kinase (PERK), activating transcription factor 6 (ATF6) and the spliced form of X-box binding protein 1 (XBP1s).10 These pathways induce the expression of the major ER heat shock proteins, including GRP94 and GRP78, that together enhance the protein folding machinery.11, 12 Once the unfolded protein response fails to control the levels of the unfolded and misfolded proteins in the ER, ER-initiated apoptotic signalling is induced along with activation of the death factor CCAAT/enhancer-binding protein homologous protein (CHOP).13 Increasing evidence has indicated that ER stress-induced trophoblastic apoptosis may be an important feature of the placental pathophysiology in PE.14, 15
Previous studies have shown that NO is a potent inducer of ER stress, thus inducing apoptosis in chondrocytes.16, 17 However, little is known about the correlation and detailed expression profiles of the NO pathway and ER-mediated apoptotic pathways in the placentas of PE patients.
To better explore the various mechanisms of severe PE, the protein expression of eNOS and iNOS as well as the overall detailed expression profiles of ER stress-related markers in the placentas of patients with PE were investigated. In addition, the apoptosis of placental trophoblasts was evaluated.
Patients and methods
Patients and sample collection
The data of women with PE (n=32) and healthy women at term (n=32) as controls undergoing a caesarean section were analysed. PE was defined as persistent systolic blood pressure ⩾140 mm Hg or diastolic blood pressure ⩾90 mm Hg respectively, with urine protein ⩾0.3 g per 24 h, occurring after 20 weeks of gestation. Severe PE was defined as new-onset systolic blood pressure ⩾160 mm Hg or diastolic blood pressure ⩾110 mm Hg, diagnosed after 20 weeks of gestation. In addition, severe PE group included cerebral or visual disturbances, right epigastric pain, pulmonary oedema, oliguria or low platelet count and abnormal liver function.18 Patients with preexisting metabolic disease or other complications were excluded. Managements were following the guidelines included antihypertensive drugs, antenatal dexamethasone treatment and timely delivery of the baby. Magnesium sulphate (MgSO4) was used in severe PE to prevent eclamptic seizures in the mother and neuroprotective for the preterm infant (<37 weeks).
All pregnant women included in this study were recruited from the Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China. The study protocol was approved by the local Ethics Committee. All patients provided written informed consent before taking part in this study. Placental tissues were biopsied a few minutes after delivery. Approximately 1 g of tissue was dissected from the central part of the maternal side of each placenta (excluding the calcified area), rinsed briefly in 0.9% saline and snap frozen in liquid nitrogen.
Western blot
Placental tissues were homogenized and lysed in radioimmunoprecipitation assay buffer supplemented with 1 mM phenylmethanesulfonyl fluoride for 1 h and then centrifuged at 15 000 r.p.m. for 30 min at 4 °C. The protein concentration was measured using a BCA protein assay kit. Equal amounts of protein (40 μg per lane) were separated on 10% SDS–polyacrylamide gels and then transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were blocked in Tris-buffered saline containing 5% nonfat milk powder for 1 h and then incubated overnight with antibodies against PERK, phospho-PERK, IRE1a, IRE1 (phospho-S724), eIF2α, phospho-eIF2a, XBP-1s, GRP78, GRP94, CHOP, caspase 4, eNOS, eNOS (phospho S1177), ATF6 and iNOS (Table 1), each diluted in Tris-buffered saline/5% nonfat milk powder; the samples were subsequently incubated with an antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control. The membranes were washed three times with Tris-buffered saline containing Tween-20 and then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:1500) for 1 h at room temperature. Proteins were detected by enhanced chemiluminescence reagents. The level of expression of each protein was analyzed using ImageJ software (US National Institutes of Health, Bethesda, MD, USA).
Immunohistochemistry
For immunohistochemistry, one full-thickness section was placed into 10% buffered formalin for 12–24 h before embedding in paraffin wax, according to standard procedures. The sections were cut at a thickness of 6 μm. After dewaxing and blocking of endogenous peroxidases, the sections were incubated with nonimmune serum for 1 h. Rabbit polyclonal antibodies against eNOS (Cell Signaling Technology, Danvers, MA, USA) and iNOS (Abcam, Cambridge, UK) were diluted 1:500 and incubated overnight at 4 °C. Sections were lightly counterstained with haematoxylin.
Detection of apoptosis by TUNEL assay
Paraffin sections were dewaxed and in situ detection of apoptosis in the renal tissues was performed by a terminal deoxynucleotidyl transferase-mediated nick-end labelling (TUNEL) assay, according to the manufacturer’s instructions (Roche Diagnostics, Indianapolis, IN, USA). After TUNEL labelling, the nucleus was labelled with 4',6-diamidino-2-phenylindole (DAPI; Molecular Probes, Invitrogen, Carlsbad, CA, USA), and the TUNEL-positive labelled cells were observed using a Nikon C2si fluorescence microscope (Nikon, Tokyo, Japan) using a 20 × objective at 1024 × 1024 resolution with 1.6 × zoom. The average number of apoptotic cells in each group was calculated by taking the average number of TUNEL-positive apoptotic cells in 10 fields from each placental sample (n=5–6/group) with × 200 magnification.
Statistical analysis
Statistical analyses were performed using SPSS software (version 20; SPSS, Chicago, IL, USA). Quantitative data were expressed as mean±s.d. The results from the three groups were compared with one-way analysis of variance or nonparametric Kruskal–Wallis tests. Multiple comparisons were assessed using Student–Newman–Keuls tests, with Bonferroni’s correction applied for post hoc analyses of qualitative data. A two-sided P-value of <0.05 was considered significant.
Results
Characteristics of the study population
A total of 64 placental samples were used for this study, including 32 controls and 32 PE placentas. All control placentas were delivered at term from normotensive healthy singleton pregnancies. The characteristics of the study population, including maternal age, prepregnancy body mass index, parity, blood pressure (systolic pressure and diastolic pressure), proteinuria (g per 24 h), uric acid, fetal gender and birth weight, are shown in Table 2. There were no significant differences in maternal age, prepregnancy body mass index, gravida, parity, aspartate transaminase level or fetal gender between the two groups. Blood pressure (systolic pressure and diastolic pressure) as well as uric acid, alanine transaminase, creatinine and urine protein levels were significantly higher in the PE group than in the normal control (P<0.05). However, the birth weight was significantly less in the PE group than in the normal group (P<0.01).
Placental expression of eNOS and iNOS in PE
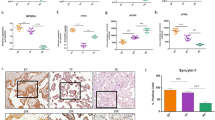
Immunohistochemistry showed strong reactivity for iNOS in PE placentas, but not in the controls. In addition, the western blot analyses indicated that the protein expression of iNOS was higher in the placentas of the PE group than in the control group. However, the expression of eNOS was less in PE placentas (P<0.05; Figure 1). Although the phosphorylation of eNOS at serine 1177 was higher in the placentas of the PE group than in the control group, the difference was not significant (P>0.05; Supplementary Figure S1).
Immunohistochemical staining and western blot analyses of eNOS and iNOS in placental tissues from normal women and those with PE (n=32 each). (a) Representative immunostaining of iNOS and eNOS (brown) in the normal and PE groups. (b) Western blot analyses of eNOS and iNOS in placental tissues from normal women and those with PE. *P<0.05 compared with a normal pregnancy.
Placental expression of ER stress marker proteins in PE
The western blot analyses showed that the expression of the proteins GRP78, GRP94, p-PERK, eIF2a, p-eIF2a, XBP1, CHOP, Ire1 and p-Ire1 was significantly higher in the placentas of women with PE than in the placentas of control women. In contrast, the levels of ATF6 and PERK were similar in the two groups (Figure 2).
Western blot analyses of ER stress markers in placental tissues from normal women and those with PE (n=32 each). Representative western blots of PERK, phospho-PERK, IRE1a, IRE1 (phospho-S724), eIF2α, phospho-eIF2a, XBP1s, GRP78, GRP94, CHOP, ATF6 and the loading control (GAPDH). Data are presented as mean±s.d. *P<0.05, PE versus control.
Increased apoptosis and caspase 4 expression in PE
According to the TUNEL staining assay, apoptosis was identified by the appearance of nuclear green staining. There were minimal apoptotic changes noted in the nuclei in the control group (Figure 3). In contrast, the placentas in the PE group revealed increased apoptosis in the chorionic trophoblasts. The apoptotic index (the percentage of TUNEL-positive nuclei per total nuclei) was statistically greater in the PE group than in the control group (15±2.7% vs 4±1.2%, P<0.05; Figure 3). Moreover, quantitation of caspase 4 by western blot indicated significantly increased expression of this protein in the PE placentas (Figures 3h–i).
Apoptosis assay with TUNEL staining and western blot analyses of caspase 4. (a) PE placenta; DAPI staining (blue). (b) PE placenta; TUNEL staining (green) indicates many apoptotic nuclei. (c) PE placenta; the merged image of TUNEL (green) and DAPI (blue) staining. (d) Control placenta; DAPI staining (blue). (e) Control placenta; TUNEL staining (green) indicates few apoptotic nuclei. (f) Control placenta; the merged image of TUNEL (green) and DAPI (blue) staining. (g) The positive ratio of TUNEL staining in PE and control placentas. (h, i) Western blot analyses of caspase 4 in placentas from normal women and those with PE (n=32 each). Scale bars=100 μm. *P<0.05, PE versus control.
Discussion
In this study, we investigated the detailed expression profiles of eNOS and iNOS as well as ER stress markers in the placentas of women with PE using western blot. The activation of NOS triggers the production of NO during pregnancy. The eNOS isoform is expressed constitutively in the vascular endothelium and maintains vascular tone through the intrinsic synthesis of NO, thereby inhibiting the adhesion of leukocytes and platelets to the endothelium and impeding the proinflammatory state. eNOS has been identified as one of the susceptible genes of PE by fine mapping.19 In addition, eNOS knockout leads to elevation of blood pressure and reduces the production of NO.20 Moreover, a significant reduction in the expression of eNOS has been observed in the endothelium of the umbilical artery of pregnant women with PE.21 Consistent with these results, lower levels of eNOS were detected by western blot analyses in the PE placentas as compared with the normal controls. Phosphorylation of eNOS at serine 1177 and at threonine 495 can modify the NO-producing activity of the coupled eNOS and the superoxide-producing activity of the uncoupled eNOS.22 We also detected the phosphorylation of eNOS at serine 1177 of placentas by western blot. However, there was no significant difference between PE group and control group, although the phosphorylation of eNOS at serine 1177 was higher in the placentas of the PE group than in the control group (Supplementary Figure S1).
iNOS is stimulated in a proinflammatory or an inflammatory condition and produces a temporary excess of NO. iNOS expression has been detected in placental tissues (within the placenta, iNOS is localized in the villous stroma and in extravillous trophoblasts23) from normal and hypertensive pregnancies.24, 25, 26 However, the relationship between iNOS and PE is still controversial.27 As inflammatory cytokines and NO have been suspected to be important contributors in the pathological process of PE,28 it is of interest to assess whether iNOS is associated with the pathogenesis of PE, particularly in pregnancy disorders caused by dyslipidaemia.
The western blot analyses showed that iNOS was increased in the placentas of women with PE, similar to previous studies.24, 29 However, these findings conflict with another report.30 Smith-Jackson et al.30 found that the protein expression of eNOS and iNOS in the placentas of women with PE was similar to control placentas by immunohistochemical staining, although eNOS mRNA expression was increased in the PE placentas. These differences may be attributed to the origin of the samples (decidual tissues and placentas, respectively). Second, the severity of PE was different in these studies; it was found that a progressive increase in eNOS staining was seen along with PE severity in the pregnant hypertensive group.21
In addition, our results were consistent with the results of the study of Ariza et al.29 that reported that placental gene expression of iNOS was significantly higher in the PE group than in the control group and eNOS expression was 67% lower in the PE group. Moreover, they observed that MgSO4 treatment presented opposite effects on eNOS and iNOS placental expression in mild PE group. However, despite the severe PE patients had been treated with MgSO4 in our study, the placental protein expression of iNOS was still higher and eNOS was lower than in the control group. This paradox may be caused by the PE of different severity in both studies. In our study, all the severe PE patients (30 of 32 patients) were treated with MgSO4 to prevent eclamptic seizures in the mother and neuroprotective for the preterm infant following the guideline from ACOG (American College of Obstetricians and Gynaecologists), but the effect of MgSO4 treatment on the placenta protein expression of iNOS and eNOS in the severe PE patients needed to be further studied.
The ER performs multiple important functions that are essential to cell survival and normal cellular function, including Ca2+ storage, post-translational modification and the folding and assembly of newly synthesized secretory proteins. A growing body of evidence has suggested recently that ER stress is involved in the pathogenesis of PE. For example, PE plus intrauterine growth restriction placentas show elevated ER stress with increased phosphorylation of eIF2α.14, 31 In addition, transmission electron microscopy has shown that the morphology of the ER is markedly altered in PE compared with normal placentas.32, 33 However, the overall expression profiles of these ER stress-related markers in patients with PE are poorly understood. Although the expression levels of PERK, ATF6 and IRE1 signalling pathway markers have been detected in PE,34 the phosphorylation of ER-related key proteins is not clear. The western blot analyses showed that the expression levels of PERK and Ire1 signalling pathway markers, including GRP78, PERK, eIF2a and CHOP, especially including p-PERK, p-eIF2a and p-Ire1, were markedly increased in the placental tissues obtained from women with PE than from those without PE. In contrast, the expression levels of the ATF6 signalling pathway markers were not significantly different in these two groups.
A recent study has suggested that the unfolded protein response pathway may be activated downstream of the innate immune system through Toll-like receptors that upregulate the expression of iNOS in macrophages.35 It has been reported that the HBsAg variant activates ER-related genes and induces ER stress that mediates the induction of reactive oxygen species, NO and apoptosis in similar manners.36 In addition, the PERK pathway is involved in NO-induced apoptosis in endothelial cells under high-glucose conditions.37 Moreover, NO-induced apoptosis in pancreatic β-cells is mediated by the ER stress pathway.38 Furthermore, the Ire1 protein has been shown to stimulate the p38 mitogen-activated protein kinase, c-Jun N-terminal kinase and nuclear factor-κB pathways, leading to the release of inflammatory cytokines by acting through tumour necrosis factor receptor-associated factor and apoptosis signal-regulating kinase 1.39 In this study, the Ire1 pathway was activated in the PE samples that may activate proinflammatory pathways and induce iNOS upregulation through its kinase domain. Moreover, serum tumour necrosis factor-α was increased in the PE group (Supplementary Figure S2). These findings are consistent with previous results indicating that PE is associated with increased inflammation.40, 41 However, in order to establish the relationship between the NO pathway and ER stress in the pathogenesis of PE, more research is needed.
Conclusion
The placental protein expression of ER stress-related markers, including GRP78, GRP94, p-PERK, eIF2a, p-eIF2a, XBP1, CHOP, Ire1, p-Ire1 and iNOS, was higher, whereas the eNOS expression was less in the PE group compared with the control group (P<0.05 for all). However, the expression of ATF6 and PERK was similar in the PE and control groups. Upregulation of CHOP and iNOS was consistent with apoptosis in the PE placenta. These data suggest that the exaggerated ER stress response and upregulated iNOS level were probably associated with increased apoptosis in the placentas of PE patients and may contribute to the pathophysiology of PE.

References
Lambert G, Brichant JF, Hartstein G, Bonhomme V, Dewandre PY . Preeclampsia: an update. Acta Anaesthesiol Belg 2014; 65: 137–149.
Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF . WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367: 1066–1074.
Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA . Placental apoptosis in preeclampsia. Obstet Gynecol 2000; 96: 271–276.
Leung DN, Smith SC, To KF, Sahota DS, Baker PN . Increased placental apoptosis in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 2001; 184: 1249–1250.
Saito S, Nakashima A . A review of the mechanism for poor placentation in early-onset preeclampsia: the role of autophagy in trophoblast invasion and vascular remodeling. J Reprod Immunol 2014; 101-102: 80–88.
Sladek SM, Magness RR, Conrad KP . Nitric oxide and pregnancy. Am J Physiol 1997; 272: R441–R463.
Kakui K, Sagawa N, Itoh H, Yura S, Korita D, Takemura M et al. Expression of nitric oxide synthase isoforms in the human placenta is not altered by labor. Endocr J 2003; 50: 535–544.
Myatt L, Eis AL, Brockman DE, Kossenjans W, Greer I, Lyall F . Inducible (type II) nitric oxide synthase in human placental villous tissue of normotensive, pre-eclamptic and intrauterine growth-restricted pregnancies. Placenta 1997; 18: 261–268.
Ron D, Walter P . Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529.
Sano R, Reed JC . ER stress-induced cell death mechanisms. Biochim Biophys Acta 2013; 1833: 3460–3470.
Malhotra JD, Kaufman RJ . The endoplasmic reticulum and the unfolded protein response. Semin Cell Dev Biol 2007; 18: 716–731.
Zhu G, Lee AS . Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol 2015; 230: 1413–1420.
Yamaguchi H, Wang HG . CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem 2004; 279: 45495–45502.
Lian IA, Loset M, Mundal SB, Fenstad MH, Johnson MP, Eide IP et al. Increased endoplasmic reticulum stress in decidual tissue from pregnancies complicated by fetal growth restriction with and without pre-eclampsia. Placenta 2011; 32: 823–829.
Zou Y, Jiang Z, Yu X, Zhang Y, Sun M, Wang W et al. MiR-101 regulates apoptosis of trophoblast HTR-8/SVneo cells by targeting endoplasmic reticulum (ER) protein 44 during preeclampsia. J Hum Hypertens 2014; 28: 610–616.
Oliver BL, Cronin CG, Zhang-Benoit Y, Goldring MB, Tanzer ML . Divergent stress responses to IL-1beta, nitric oxide, and tunicamycin by chondrocytes. J Cell Physiol 2005; 204: 45–50.
Takada K, Hirose J, Yamabe S, Uehara Y, Mizuta H . Endoplasmic reticulum stress mediates nitric oxide-induced chondrocyte apoptosis. Biomed Rep 2013; 1: 315–319.
Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM, Druzin M et al. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122: 1122–1131.
Guo G, Lade JA, Wilton AN, Moses EK, Grehan M, Fu Y et al. Genetic susceptibility to pre-eclampsia and chromosome 7q36. Hum Genet 1999; 105: 641–647.
Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001; 104: 342–345.
Bhavina K, Radhika J, Pandian SS . VEGF and eNOS expression in umbilical cord from pregnancy complicated by hypertensive disorder with different severity. Biomed Res Int 2014; 2014: 982159.
Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S et al. eNOS activation and NO function: structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol 2011; 210: 271–284.
Thomson AJ, Telfer JF, Kohnen G, Young A, Cameron IT, Greer IA et al. Nitric oxide synthase activity and localization do not change in uterus and placenta during human parturition. Hum Reprod 1997; 12: 2546–2552.
Schiessl B, Mylonas I, Hantschmann P, Kuhn C, Schulze S, Kunze S et al. Expression of endothelial NO synthase, inducible NO synthase, and estrogen receptors alpha and beta in placental tissue of normal, preeclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem 2005; 53: 1441–1449.
Faxen M, Nisell H, Kublickiene KR . Altered mRNA expression of ecNOS and iNOS in myometrium and placenta from women with preeclampsia. Arch Gynecol Obstet 2001; 265: 45–50.
Schiessl B, Mylonas I, Kuhn C, Kunze S, Schulze S, Friese K et al. Expression of estrogen receptor-alpha, estrogen receptor-beta and placental endothelial and inducible NO synthase in intrauterine growth-restricted and normal placentals. Arch Med Res 2006; 37: 967–975.
Yoshida T, Limmroth V, Irikura K, Moskowitz MA . The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab 1994; 14: 924–929.
Moffett A, Hiby SE . How Does the maternal immune system contribute to the development of pre-eclampsia? Placenta 2007; 28 (Suppl A): S51–S56.
Ariza AC, Bobadilla N, Diaz L, Avila E, Larrea F, Halhali A . Placental gene expression of calcitonin gene-related peptide and nitric oxide synthases in preeclampsia: effects of magnesium sulfate. Magnes Res 2009; 22: 44–49.
Smith-Jackson K, Hentschke MR, Poli-de-Figueiredo CE, Pinheiro da Costa BE, Kurlak LO, Broughton Pipkin F et al. Placental expression of eNOS, iNOS and the major protein components of caveolae in women with pre-eclampsia. Placenta 2015; 36: 607–610.
Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS et al. Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 2008; 173: 451–462.
Burton GJ, Yung HW . Endoplasmic reticulum stress in the pathogenesis of early-onset pre-eclampsia. Pregnancy Hypertens 2011; 1: 72–78.
Jones CJ, Fox H . An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta 1980; 1: 61–76.
Fu J, Zhao L, Wang L, Zhu X . Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan J Obstet Gynecol 2015; 54: 19–23.
Martinon F, Chen X, Lee AH, Glimcher LH . TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 2010; 11: 411–418.
Lee IK, Lee SA, Kim H, Won YS, Kim BJ . Induction of endoplasmic reticulum-derived oxidative stress by an occult infection related S surface antigen variant. World J Gastroenterol 2015; 21: 6872–6883.
Zhang X, Fu Y, Xu X, Li M, Du L, Han Y et al. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide 2014; 40: 10–16.
Oyadomari S, Takeda K, Takiguchi M, Gotoh T, Matsumoto M, Wada I et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA 2001; 98: 10845–10850.
Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS . Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta 2009; 30 (Suppl A): S43–S48.
Takagi Y, Nikaido T, Toki T, Kita N, Kanai M, Ashida T et al. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch 2004; 444: 49–55.
Teran E, Escudero C, Moya W, Flores M, Vallance P, Lopez-Jaramillo P . Elevated C-reactive protein and pro-inflammatory cytokines in Andean women with pre-eclampsia. Int J Gynaecol Obstet 2001; 75: 243–249.
Acknowledgements
This work was supported by the National Natural Science Foundation (No. 81302399 and 81370775) and China Postdoctoral Science Foundation (No.2012M521587 and 2014T70798). This work was also supported by Guangdong Natural Science Foundation (No. S2013040013853), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20114423110004) and Key Technology R&D Program of Guangzhou Science and Technology Bureau (11A52021213). We thank the Biobank of The Third Affiliated Hospital of Guangzhou Medical University for samples support. We also thank Medjaden Bioscience Limited for its linguistic assistance to edit this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Du, L., He, F., Kuang, L. et al. eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J Hum Hypertens 31, 49–55 (2017). https://doi.org/10.1038/jhh.2016.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2016.17
This article is cited by
-
Methyltransferase-like 3 aggravates endoplasmic reticulum stress in preeclampsia by targeting TMBIM6 in YTHDF2-dependent manner
Molecular Medicine (2023)
-
Elevated human placental heat shock protein 5 is associated with spontaneous preterm birth
Pediatric Research (2023)
-
Regulation of renal nitric oxide and eNOS/iNOS expression by tadalafil participates in the mitigation of amphotericin B–induced renal injury: Down-regulation of NF-κB/iNOS/caspase-3 signaling
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
CircRNA_0088196 Regulates Trophoblast Proliferation and Apoptosis in Preeclampsia Through the miR-379-5p/HSPA5 Axis
Biochemical Genetics (2023)
-
Downregulation of LINC00221 promotes angiopoiesis in HUVEC and inhibits recruitment of macrophages by augmenting miR-542-3p in trophoblast cells
Journal of Assisted Reproduction and Genetics (2022)