Abstract
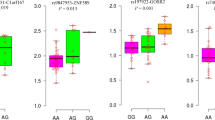
In this study, we determined the association of 1180 non-synonymous single-nucleotide polymorphisms (SNPs) with systolic blood pressure (SBP) and hypertensive status. A total of 8842 subjects were taken from two community-based cohorts—Ansung (n=4183) and Ansan (n=4659), South Korea—which had been established for genome-wide association studies (GWAS). Five SNPs (rs16835244, rs2286672, rs6265, rs17237198 and rs7312017) were significantly associated (P-values: 0.003–0.0001, not corrected for genome-wide significance) with SBP in both cohorts. Of these SNPs, rs16835244 and rs2286672 correlated with risk for hypertension. The rs16835244 SNP replaces Ala288 in arginine decarboxylase (ADC) with serine, and rs2286672 replaces Arg172 in phospholipase D2 (PLD2) with cysteine. A comparison of peptide sequences between vertebrate homologues revealed that the SNPs identified occur at conserved amino-acid residues. In silico analysis of the protein structure showed that the substitution of a polar residue, serine, for a non-polar alanine at amino-acid residue 288 affects a conformational change in ADC, and that Arg172 in PLD2 resides in the PX domain, which is important for membrane trafficking. These results provide insights into the function of these non-synonymous SNPs in the development of hypertension. The study investigating non-synonymous SNPs from GWAS not only by statistical association analysis but also by biological relevance through the protein structure might be a good approach for identifying genetic risk factors for hypertension, in addition to discovering causative variations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garcia EA, Newhouse S, Caulfield MJ, Munroe PB . Genes and hypertension. Curr Pharm Des 2003; 9 (21): 1679–1689.
Whitworth JA . 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21 (11): 1983–1992.
Whelton PK . Epidemiology of hypertension. Lancet 1994; 344 (8915): 101–106.
Gong M, Hubner N . Molecular genetics of human hypertension. Clin Sci (Lond) 2006; 110 (3): 315–326.
Dickson ME, Sigmund CD . Genetic basis of hypertension: revisiting angiotensinogen. Hypertension 2006; 48 (1): 14–20.
Staessen JA, Wang JG, Ginocchio G, Petrov V, Saavedra AP, Soubrier F et al. The deletion/insertion polymorphism of the angiotensin converting enzyme gene and cardiovascular-renal risk. J Hypertens 1997; 15 (12 Part 2): 1579–1592.
Herrmann SM, Nicaud V, Tiret L, Evans A, Kee F, Ruidavets JB et al. Polymorphisms of the beta2 -adrenoceptor (ADRB2) gene and essential hypertension: the ECTIM and PEGASE studies. J Hypertens 2002; 20 (2): 229–235.
Saavedra JM . Studies on genes and hypertension: a daunting task. J Hypertens 2005; 23 (5): 929–932.
WTCCC. Genome-wide association study of 14 000 cases of seven common diseases and 3000 shared controls. Nature 2007; 447 (7145): 661–678.
Ehret GB, Morrison AC, O’Connor AA, Grove ML, Baird L, Schwander K et al. Replication of the wellcome trust genome-wide association study of essential hypertension: the family blood pressure program. Eur J Hum Genet 2008; 16 (12): 1507–1511.
Hong KW, Jin HS, Cho YS, Lee JY, Lee JE, Cho NH et al. Replication of the wellcome trust genome-wide association study on essential hypertension in a Korean population. Hypertens Res 2009; 32 (7): 570–574.
Ioannidis JP, Thomas G, Daly MJ . Validating, augmenting and refining genome-wide association signals. Nat Rev Genet 2009; 10 (5): 318–329.
Wang Y, O’Connell JR, McArdle PF, Wade JB, Dorff SE, Shah SJ et al. From the cover: whole-genome association study identifies STK39 as a hypertension susceptibility gene. Proc Natl Acad Sci USA 2009; 106 (1): 226–231.
Org E, Eyheramendy S, Juhanson P, Gieger C, Lichtner P, Klopp N et al. Genome-wide scan identifies CDH13 as a novel susceptibility locus contributing to blood pressure determination in two European populations. Hum Mol Genet 2009; 18 (12): 2288–2296.
Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L et al. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 2009; 41 (6): 666–676.
Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, Dehghan A et al. Genome-wide association study of blood pressure and hypertension. Nat Genet 2009; 41 (6): 677–687.
Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ et al. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 2009; 41 (5): 527–534.
Rabbee N, Speed TP . A genotype calling algorithm for affymetrix SNP arrays. Bioinformatics 2006; 22 (1): 7–12.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81 (3): 559–575.
Jung J, Lee B . Protein structure alignment using environmental profiles. Protein Eng 2000; 13 (8): 535–543.
Kraulis PJ . MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J Appl Cryst 1991; 24: 946–950.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21 (2): 263–265.
Albeck S, Dym O, Unger T, Snapir Z, Bercovich Z, Kahana C . Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci 2008; 17 (5): 793–802.
Regunathan S, Reis DJ . Stimulation of imidazoline receptors inhibits proliferation of human coronary artery vascular smooth muscle cells. Hypertension 1997; 30 (2 Part 1): 295–300.
Cobos-Puc LE, Villalon CM, Ramirez-Rosas MB, Sanchez-Lopez A, Lozano-Cuenca J, Gomez-Diaz B et al. Pharmacological characterization of the inhibition by moxonidine and agmatine on the cardioaccelerator sympathetic outflow in pithed rats. Eur J Pharmacol 2009; 616 (1–3): 175–182.
Mukaddam-Daher S, Menaouar A, Paquette PA, Jankowski M, Gutkowska J, Gillis MA et al. Hemodynamic and cardiac effects of chronic eprosartan and moxonidine therapy in stroke-prone spontaneously hypertensive rats. Hypertension 2009; 53 (5): 775–781.
Rouch AJ, Kudo LH . Agmatine inhibits arginine vasopressin-stimulated urea transport in the rat inner medullary collecting duct. Kidney Int 2002; 62 (6): 2101–2108.
Gao Y, Gumusel B, Koves G, Prasad A, Hao Q, Hyman A et al. Agmatine: a novel endogenous vasodilator substance. Life Sci 1995; 57 (8): PL83–PL86.
Sun MK, Regunathan S, Reis DJ . Cardiovascular responses to agmatine, a clonidine-displacing substance, in anesthetized rat. Clin Exp Hypertens 1995; 17 (1–2): 115–128.
Gerova M, Torok J . Hypotensive effect of agmatine, arginine metabolite, is affected by NO synthase. Physiol Res 2004; 53 (4): 357–363.
Du G, Huang P, Liang BT, Frohman MA . Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol Biol Cell 2004; 15 (3): 1024–1030.
Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS et al. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 2006; 290 (1): R96–R104.
Ferguson AV, Washburn DL, Latchford KJ . Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001; 226 (2): 85–96.
Yu H, Zhang Z, Shi Y, Bai F, Xie C, Qian Y et al. Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry 2008; 69 (7): 1104–1111.
Chen CM, Chen IC, Chang KH, Chen YC, Lyu RK, Liu YT et al. Nuclear receptor NR4A2 IVS6 +18insG and brain derived neurotrophic factor (BDNF) V66M polymorphisms and risk of Taiwanese Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet 2007; 144B (4): 458–462.
Rybakowski JK . BDNF gene: functional Val66Met polymorphism in mood disorders and schizophrenia. Pharmacogenomics 2008; 9 (11): 1589–1593.
Jiang H, Wang R, Liu Y, Zhang Y, Chen ZY . BDNF Val66Met polymorphism is associated with unstable angina. Clin Chim Acta 2009; 400 (1–2): 3–7.
Lewin GR, Barde YA . Physiology of the neurotrophins. Annu Rev Neurosci 1996; 19: 289–317.
Donovan MJ, Miranda RC, Kraemer R, McCaffrey TA, Tessarollo L, Mahadeo D et al. Neurotrophin and neurotrophin receptors in vascular smooth muscle cells. Regulation of expression in response to injury. Am J Pathol 1995; 147 (2): 309–324.
Acknowledgements
This study was supported by a grant from the Kyung Hee University to B.O. (KHU-20090597). The Consortium for Large Scale Genome Wide Association Study was supported by genotyping data (Genome Wide association analysis of a community-based cohort study; 2007) from the Korean Genome Analysis Project (4845-301) from the Korea National Institute of Health (Korea Center for Disease Control, Ministry for Health, Welfare and Family Affairs), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Journal of Human Hypertension website
Supplementary information
Rights and permissions
About this article
Cite this article
Hong, KW., Jin, HS., Lim, JE. et al. Non-synonymous single-nucleotide polymorphisms associated with blood pressure and hypertension. J Hum Hypertens 24, 763–774 (2010). https://doi.org/10.1038/jhh.2010.9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhh.2010.9
Keywords
This article is cited by
-
Phospholipase D1 regulation of TNF-alpha protects against responses to LPS
Scientific Reports (2018)
-
Phospholipase D2 loss results in increased blood pressure via inhibition of the endothelial nitric oxide synthase pathway
Scientific Reports (2017)
-
A nonsynonymous SNP in BANK1 is associated with serum LDL cholesterol levels in three Korean populations
Journal of Human Genetics (2015)
-
The Role of Phospholipase D1 in Liver Fibrosis Induced by Dimethylnitrosamine In Vivo
Digestive Diseases and Sciences (2014)
-
Computational Study of ADD1 Gene Polymorphism Associated with Hypertension
Cell Biochemistry and Biophysics (2013)