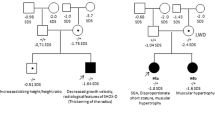
Abstract
Heterozygous aberrations of SHOX gene have been reported to be responsible for Léri–Weill dyschondrosteosis (LWD) and small portion of idiopathic short stature. The study was established to assess effectiveness of using phenotype ‘scoring form’ in patients indicated for SHOX gene defect analysis. The submitted study is based on a retrospective group of 352 unrelated patients enrolled as a part of the routine diagnostic practice and analyzed for aberrations affecting the SHOX gene. All participants were scanned for deletion/duplication within the main pseudoautosomal region (PAR1) using the multiplex ligation-dependent probe amplification (MLPA) method. The phenotype ‘scoring form’ is used in our laboratory practice to preselect patients for subsequent mutation analysis of SHOX gene-coding sequences. The overall detection rate was 11.1% but there was a significant increase in frequency of SHOX gene defect positive with increasing achieved score (P<0.0001). The most frequent aberration was a causal deletion within PAR1. In three probands, MLPA analysis indicated a more complex rearrangement. Madelung deformity or co-occurrence of disproportionate short stature, short forearm and muscular hypertrophy had represented the most potent markers to determine the likelihood of SHOX gene defect detection. We conclude that appliance of phenotype ‘scoring form’ had saved excessive sample analysis and enabled effective routine diagnostic testing.
Similar content being viewed by others
Introduction
Heterozygous aberration of SHOX gene (Short stature HOmeoboX; ID 6473) or deletion/duplication of one of its numerous enhancers have been reported to be responsible for Léri–Weill dyschondrosteosis (LWD; MIM 127300) and small portion of idiopathic short stature (MIM 300582).1, 2 There is a phenotype variability within afflicted family members ranging from small stature (frequently disproportionate) to a fully expressed LWD phenotype. It is partly due to estrogen effect, still it means a thin line between both of these phenotypes. Homozygous defect of SHOX gene is responsible for a rare Langer mesomelic dysplasia (MIM 249700) characterized with more severe phenotype. Haploinsufficiency of SHOX in LWD patients is associated with variable degrees of growth impairment with or without a spectrum of skeletal abnormalities.
SHOX gene is situated within the main pseudoautosomal region (PAR1; 2.7 Mb) on the p arm of sex chromosomes and its protein product acts as an important transcription factor during limb development.3 Besides the limb bud, there is a significant expression of the SHOX gene within the first and second pharyngeal arches mesenchyma.4 Spatio-temporal fine-tuning of SHOX gene expression is regulated by multiple mechanisms.5, 6, 7, 8
Our study summarizes experiences concerning genetic analysis of patients suspected of LWD collected in the course of routine laboratory practice. The ‘scoring form’ of clinical criteria evaluating likelihood of SHOX gene defect established on the original Rappold’s scoring9 is an integral part of medical indication for the relevant molecular analysis in our laboratory routine. Our study is a retrospective type of study where no other criteria except for medical indication (for SHOX gene analysis) made by local medical specialists (endocrinologists and clinical geneticists as well as pediatricians) were established. The ‘scoring form’ is assumed to help these specialists decide if the SHOX gene aberration is under consideration. Even though not all forms are filled out correctly and completely, we have collected a representative cohort of patients with sufficient clinical data tested for PAR1 deletion/duplication and SHOX gene-coding sequences mutation. Numerous publications have proofed strong association of SHOX gene haploinsufficiency with LWD phenotype; however, strictly selected project participants are often indicated by well-trained specialist. Our not so strictly selected cohort of patients was indicated by numerous local medical specialists and generally represents individuals with more variable phenotype. The main purpose of the study was to evaluate the effectiveness of using ‘scoring form’ in patients indicated for SHOX gene defect analysis and to establish whether selected phenotypic markers remain valid even in such a cohort of patients.
Materials and methods
Subjects
Our study is based on a retrospective group of patients from the database of the Institute of Biology and Medical Genetics, General University Hospital and The First Faculty of Medicine of Charles University in Prague (IBMG) in close cooperation with other medical genetic, endocrinology and pediatric departments. Participants were enrolled as part of the routine diagnostic practice. Indication for molecular analysis of SHOX gene defects is possible only on the basis of prior medical specialist examination (mostly endocrinologist or clinical geneticist) in the Czech Republic. We highly recommend the completed phenotype ‘scoring form’ (Table 1) enclosed with such routine indication as well as information regarding height and mean height s.d. score of proband and height of both parents if possible. All patients introduced to the study (or their legal representatives) signed an informed consent form for blood withdrawal and DNA analysis.
Methods
All participants (352 unrelated probands) were screened for PAR1 deletion/duplication using the Multiplex Ligation-Dependent Probe Amplification (MLPA) Kit P018-G1 (or its older version B; D1; E1; F1) SHOX probemix (MRC-Holland, Amsterdam, The Netherlands).10
The MLPA reaction was run with 50–150 ng of DNA, according to the manufacturer’s instructions with subsequent fragmentation analysis conducted on an ABI PRISM 310 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Both visual and computational evaluation was performed for each sample against the run negative control. The peak areas were normalized according to the instructions of the manufacturer. A personally constructed Microsoft Excel (Microsoft, Redmond, WA, USA) table was used for the entry of all of these calculations. Probands suspected of X chromosome mosaicism were analyzed by the use of Devyser Resolution XY v2 Kit (Devyser AB, Hägersten, Sweden). Only those patients who reached the score of at least 4 in the ‘scoring form’ (168 patients) were further assigned for mutation analysis of the coding portion of SHOX gene (exons 2–6a/6b) employing the direct Sanger sequencing method. Sequencing reaction was prepared according to the manufacturer’s instructions with the use of BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) and ran on an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems). Primers and amplification conditions are indicated elsewhere.11 Statistical comparisons were performed using Fisher exact tests for categorical variables at a two‐sided P-value (STATISTICA Ver 9.1) (StatSoft, Inc., Dell Software Inc., Aliso Viejo, CA, USA, 2010).
Results
The overall detection rate based on 352 unrelated probands was 11.1%. The most frequent was deletion encompassing the SHOX gene (46.2%), followed by SHOX gene sequence mutation (17.9%) and exclusive deletion of downstream enhancer elements (12.8%). An overview of detected SHOX gene sequence mutations and polymorphisms is summarized in Table 2. Besides, duplication of downstream enhancer elements (10.3%), single SHOX gene duplication and single duplication of upstream enhancer element (CNE-2) have occurred. In two female patients, there was a 68–75% reduction of all probes focusing on PAR1 and PAR2 as well as on X-specific regions. This reduction is slightly higher than expected for complete deletion of X chromosome. Although both female probands display disproportionate short stature (height −2.5 s.d.) combined with short forearm and muscular hypertrophy, they are fertile and lack other features typical for Turner syndome. We thus hypothesized a mosaicism with partial cell line lacking X chromosome. We used the Devyser Resolution XY Kit to support our hypothesis. There was a clear imbalance of peak areas of heterozygous markers and the portion of XO cell line was estimated to be approximately 60% in both cases.
In one male proband, the MLPA analysis detected duplication of all PAR1 probes as well as deletion of PAR2 probe and absence of both Yq control probes. The suggested rearrangement was the isochromosome Yp (iYp).
The extent of detected PAR1 deletions/duplications was highly variable and there was not a significant recombination hotspot within this region (Figure 1).
Scheme indicating breakpoints of microdeletions and microduplications within PAR1 evaluated on the basis of SALSA MLPA P018 probemixes (various versions) in our cohort of probands (P). Placing of SHOX gene as well as upstream and downstream evolutionarily conserved non-coding elements (CNEs) acting as SHOX enhancers are depicted.
Several non-pathogenic rearrangements were observed. There was a recurrent duplication of ASMT gene probe (MLPA Kit P018-G1, probe L00712) with frequency of 2.3% and common small PAR1 ~4.9 kb deletion (MLPA Kit P018-D1, probe L05101) some 200 kb downstream of SHOX gene. Moreover, four unique deletions and four duplications of various extent and localization within the PAR1 region were detected with no obvious association with the considered phenotype.
The following data were obtained from the subgroup of 247 unrelated individuals with sufficiently completed ‘scoring form’. In 168 of them was the score of ⩾4. Detection rate of SHOX gene defects in the group with score <4 points (low-scoring group) was 3.8%, which significantly differs (P=0.0013) from the detection rate of 18.5% in the group of individuals with score of ⩾4 (high-scoring group). Such difference could not be caused by the absence of SHOX gene mutation analysis in the low-scoring group as such mutations are quite rare and represent only a small fraction (22.6%) of SHOX gene defects detected in the high-scoring group. Patients with score <4 points covered 32.0% of all the assessed indications. The PAR1 aberrations in the low-scoring group comprised of deletion/duplication of downstream regulatory sequences and of a carrier with the iYp rearrangement. For further evaluation, the subgroup of 247 individuals was divided into following categories according to the achieved score: 0 points, 1–3 points, 4–10 points, 11–15 points, and ⩾16 points. There was no significant difference in proportion of men and women in each category as well as in average height s.d. (~2.56) and median height s.d. (~2.59). The first category (0 points) represents patients indicated primarily on the presence of isolated short stature (frequently with family history). Detection rate for individual categories is indicated in Figure 2.
It is obvious that the ‘scoring form’ was helpful in selecting patients with higher probability of SHOX defect. The pronounced increase of positive patients with last category (⩾16 points) is due to the presence of Madelung deformity either in the proband or in a first-degree relative. Madelung deformity still represents the most potent marker for evaluation of probability of SHOX gene defect detection that is evident from the frequency of selected clinical features among patients with and without detected SHOX locus aberration listed in Table 3. Besides Madelung deformity, a co-occurrence of disproportionate short stature, short forearm and appearance of muscular hypertrophy is present in 59.3% of positive patients compared with 14.3% of SHOX gene defect negative.
Discussion
Recombination rate in PAR1 is consistent with one obligatory crossover per male meiosis having a crossover rate 17-fold greater than the genome-wide average.12 High resolution sperm-typing measured a single recombination hotspot within the SHOX gene.13 All of it results in a high frequency of rearrangements of both pathogenic and non-pathogenic within the PAR1 region. Duplication of ASMT gene is mainly discussed as a risk factor for autism spectrum disorders; to our knowledge, none of these patients had a history of this condition.14 Frequency of a common small ~4.9 kb PAR1 rearrangement was determined to be 13.1% in the Czech population.15 There was not a significant difference between its population frequency and frequency in patients with idiopathic short stature. There is a quite high variability of SHOX defect detection rate among different studies of patients with short stature;2, 11, 16, 17 it is partly because of different study inclusion criteria; however, population-specific dependencies could exist. The inclusion criteria for our study were very flexible and thus the lower overall incidence of SHOX defects in our sample (11.1%) is consistent.
Only a small portion of patients with diagnosed short stature have aberration of SHOX gene. It is highly desirable to narrow down the number of investigated patients by using the appropriate phenotype scoring system. We had introduced such ‘scoring form’ in our laboratory practice, based on the original Rappold’s scoring system, to preselect patients intended for advanced SHOX gene mutation analysis. Our ‘scoring form’ was purposely modified, including high score for Madelung deformity (6 points), what reflects its high informative potential and no body mass index criteria inclusion. High body mass index in SHOX defect positives is mostly due to disproportionate short stature and muscular hypertrophy; both of these symptoms are already included in our ‘scoring form’, and moreover, significance of body mass index would fade with higher obese children in our population (special report from the The National Institute of Public Health of the Czech Republic: The change in body proportions, prevalence of overweight and obesity; www.szu.cz).
There was an obvious increase in frequency of SHOX gene defect positives with increasing achieved score. Madelung deformity or co-occurrence of disproportionate short stature, short forearm and muscular hypertrophy had represented the most potent markers to determine likelihood of SHOX gene defect detection.
We can conclude that phenotype ‘scoring form’ appliance had saved excessive sample analysis and enabled effective routine diagnostic testing.
References
Gatta, V., Palka, C., Chiavaroli, V., Franchi, S., Cannataro, G., Savastano, M. et al. Spectrum of phenotypic anomalies in four families with deletion of the SHOX enhancer region. BMC Med. Genet. 15, 87 (2014).
Jorge, A. A., Souza, S. C., Nishi, M. Y., Billerbeck, A. E., Liborio, D. C., Kim, C. A. et al. SHOX mutations in idiopathic short stature and Leri-Weill dyschondrosteosis: frequency and phenotypic variability. Clin. Endocrinol. (Oxf.) 66, 130–135 (2007).
Munns, C. J., Haase, H. R., Crowther, L. M., Hayes, M. T., Blaschke, R., Rappold, G. et al. Expression of SHOX in human fetal and childhood growth plate. J. Clin. Endocrinol. Metab. 89, 4130–4135 (2004).
Clement-Jones, M., Schiller, S., Rao, E., Blaschke, R. J., Zuniga, A., Zeller, R. et al. The short stature homeobox gene SHOX is involved in skeletal abnormalities in Turner syndrome. Hum. Mol. Genet. 9, 695–702 (2000).
Fukami, M., Kato, F., Tajima, T., Yokoya, S. & Ogata, T. Transactivation function of an approximately 800-bp evolutionarily conserved sequence at the SHOX 3′ region: implication for the downstream enhancer. Am. J. Hum. Genet. 78, 167–170 (2006).
Durand, C., Bangs, F., Signolet, J., Decker, E., Tickle, C. & Rappold, G. Enhancer elements upstream of the SHOX gene are active in the developing limb. Eur. J. Hum. Genet. 18, 527–532 (2010).
Durand, C., Roeth, R., Dweep, H., Vlatkovic, I., Decker, E., Schneider, K. U. et al. Alternative splicing and nonsense-mediated RNA decay contribute to the regulation of SHOX expression. PLoS ONE 6, e18115 (2011).
Ge, Y. & Porse, B. T. The functional consequences of intron retention: alternative splicing coupled to NMD as a regulator of gene expression. Bioessays 36, 236–243 (2014).
Rappold, G., Blum, W. F., Shavrikova, E. P., Crowe, B. J., Roeth, R., Quigley, C. A. et al. Genotypes and phenotypes in children with short stature: clinical indicators of SHOX haploinsufficiency. J. Med. Genet. 44, 306–313 (2007).
Schouten, J. P., McElgunn, C. J., Waaijer, R., Zwijnenburg, D., Diepvens, F. & Pals, G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 30, e57 (2002).
Hirschfeldova, K., Solc, R., Baxova, A., Zapletalova, J., Kebrdlova, V., Gaillyova, R. et al. SHOX gene defects and selected dysmorphic signs in patients of idiopathic short stature and Leri-Weill dyschondrosteosis. Gene 491, 123–127 (2012).
Schmitt, K., Lazzeroni, L. C., Foote, S., Vollrath, D., Fisher, E. M., Goradia, T. M. et al. Multipoint linkage map of the human pseudoautosomal region, based on single-sperm typing: do double crossovers occur during male meiosis? Am. J. Hum. Genet. 55, 423–430 (1994).
May, C. A., Shone, A. C., Kalaydjieva, L., Sajantila, A. & Jeffreys, A. J. Crossover clustering and rapid decay of linkage disequilibrium in the Xp/Yp pseudoautosomal gene SHOX. Nat. Genet. 31, 272–275 (2002).
Veatch, O. J., Goldman, S. E., Adkins, K. W. & Malow, B. A. Melatonin in children with autism spectrum disorders: how does the evidence fit together? J. Nat. Sci. 1, e125 (2015).
Solc, R., Hirschfeldova, K., Kebrdlova, V. & Baxova, A. Analysis of common SHOX gene sequence variants and ~4.9-kb PAR1 deletion in ISS patients. J. Genet. 93, 505–508 (2014).
Rosilio, M., Huber-Lequesne, C., Sapin, H., Carel, J. C., Blum, W. F. & Cormier-Daire, V. Genotypes and phenotypes of children with SHOX deficiency in France. J. Clin. Endocrinol. Metab. 97, E1257–E1265 (2012).
Rappold, G. A., Fukami, M., Niesler, B., Schiller, S., Zumkeller, W., Bettendorf, M. et al. Deletions of the homeobox gene SHOX (short stature homeobox) are an important cause of growth failure in children with short stature. J. Clin. Endocrinol. Metab. 87, 1402–1406 (2002).
Acknowledgements
We thank the following doctors for prolonged collaboration: Eva Al Taji, Alice Baxova, Rastislav Beharka, Andrea Gregorova, Stanislava Kolouskova, Jan Lebl, Barbora Obermannova, Ales Panczak, Ivana Plasilova, Sarka Prasilova, Stepanka Pruhova, Antonin Sipek, Jana Soukalova, Zdenek Sumnik, Jirina Zapletalova, and Jana Zidovska.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hirschfeldova, K., Florianova, M., Kebrdlova, V. et al. Detection of SHOX gene aberrations in routine diagnostic practice and evaluation of phenotype scoring form effectiveness. J Hum Genet 62, 253–257 (2017). https://doi.org/10.1038/jhg.2016.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2016.117
This article is cited by
-
Screening of the SHOX/PAR1 region using MLPA and miRNA expression profiling in a group of Egyptian children with non-syndromic short stature
Egyptian Journal of Medical Human Genetics (2020)
-
Skeletal Dysplasias: What Every Bone Health Clinician Needs to Know
Current Osteoporosis Reports (2017)