Abstract
Three recessive mutations in the sodium leak channel, nonselective (NALCN) have been reported to cause intellectual disability and hypotonia. In addition, 14 de novo heterozygous mutations have been identified in 15 patients with arthrogryposis and neurodevelopmental impairment. Here, we report three patients with neurodevelopmental disease and hypotonia, harboring one recurrent (p.R1181Q) and two novel mutations (p.L312V and p.V1020F) occurring de novo in NALCN. Mutation p.L312 is located in the pore forming S6 region of domain I and p.V1020F in the S5 region of domain III. Mutation p.R1181Q is in a linker region. Mapping these three mutations to a model of NALCN showed p.Leu312 and p.Val1020 positioned in the hydrophobic core of the pore modules, indicating these two mutations may affect the gating function of NALCN. Although p.R1181Q is unlikely to affect the ion channel structure, previous studies have shown that an analogous mutation in Caenorhabditis elegans produced a phenotype with a coiling locomotion, suggesting that p.R1181Q could also affect NALCN function. Our three patients showed profound intellectual disability and growth delay, facial dysmorphologies and hypotonia. The present data support previous work suggesting heterozygous NALCN mutations lead to syndromic neurodevelopmental impairment.
Similar content being viewed by others
Introduction
The 4 × 6 transmembrane channel family members contain four homologous repeat domains (I–IV) and six transmembrane segments (S1–S6) in each domain.1, 2, 3 The sodium leak channel, nonselective (NALCN) is one of the 4 × 6 transmembrane channel family members, and it encodes voltage-independent nonselective cation channels.1, 2, 3 NALCN is widely expressed in the central nervous system, especially in neurons, and is responsible for half of the background sodium leak current and the regulation of neuronal excitabilities in vertebrates.1, 4 NALCN mutations have been linked to human diseases.5, 6, 7, 8 Recessive (homozygous) NALCN mutations (p.Y497Tfs*21,6 p.W1287L,6 and p.Q642X5) were reported in children from three consanguineous families showing global developmental delay and hypotonia.5, 6 p.Q642X was detected in two siblings (one female and one male) from a family with infantile neuroaxonal dystrophy.5 p.W1287L located outside of pore-forming domains was found in three female patients affected with mild hypotonia, speech impairment, cognitive delay and easily controlled seizures,6 and p.Y497Tfs*21 was found in three familial male patients with severe hypotonia, speech impairment and cognitive delay.6 Conversely, 14 dominant NALCN mutations (occurring de novo) were recently found in patients with either distal arthrogryposis (congenital contractures of the limbs and face), hypotonia, and global developmental delay (CLIFAHDD) syndrome or intellectual disability, ataxia and arthrogryposis.9, 10 The mutations were located in or near the pore forming regions of NALCN, some of the patients’ phenotypes were commonly observed. We report three patients with de novo NALCN mutations and discuss genotype–phenotype correlations.
Materials and methods
Genomic DNA was extracted from the peripheral blood of patients and their parents. Whole exome sequencing was performed as previously described.11 In brief, approximately 3 μg DNA was sheared and used for a SureSelect Human All Exon V5 library (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions (method as previously described11). All variants within exons or ±30 bp from exon–intron boundaries, those registered in dbSNP137 (minor allele frequency ⩾0.01), the National Heart Lung and Blood Institute Exome Sequencing Project Exome Variant Server (NHLBI-ESP 6500, http://evs.gs.washington.edu/EVS/) and our in-house databases (exome data from 575 Japanese individuals) were removed. Variants were confirmed by Sanger sequencing using an ABI PRISM 3500xl autosequencer (Life Technologies, Carlsbad, CA, USA). In this analysis, NALCN variants were annotated according to NCBI reference sequence NM_052867.2, and amino acid positions are based on NP_443099.1. To confirm NALCN expression in the central nervous system, we performed TaqMan quantitative real-time PCR analysis with cDNAs of adult human tissues (Human MTC Panel I, #636742) (Clontech Laboratories, Mountain View, CA, USA), fetal human tissues (Human Fetal MTC Panel, #636747) (Clontech Laboratories) as a template. Predesigned TaqMan probes for human NALCN (Hs01075987_m1 and Hs00380168_m1), and human β-actin (ACTB, 4326315E) were purchased from Life Technologies. This study was approved by the Institutional Review Board of Yokohama City University School of Medicine. Written informed consent was obtained from patients or parents of pediatric patients.
Results and discussion
Three de novo NALCN mutations were identified: c.934C>G (p.L312V), c.3542G>A (p.V1020F) and c.3058G>T (p.R1181Q) in three unrelated patients with developmental delay (Table 1 and Figure 1). Three de novo missense mutations were detected and predicted as pathogenic by SIFT,12 PolyPhen2,13 and MutationTaster,14 and mutations occurred at evolutionarily conserved amino acids among different species (Table 1).
Genetic and structural depiction of NALCN mutations. (a) Missense mutations in NALCN found in the current study (red dots), a study by Chong et al.9 (blue dots) and Aoyagi et al.10 (green dots). Squares reflect autosomal recessive mutations from previous reports. A modeled structure of human NALCN is shown, viewed from the intracellular side of the membrane (b) and from the membrane plane (c), with indication of the found mutation positions. The modeled structures of domain I, III and IV (DI, DIII and DIV) were constructed individually based on the crystal structures of a cAMP-bound potassium channel from the bacterium Mesorhizobium loti (PDB code 4chw),17 a voltage-gated Na+-channel from Arcobacter butzleri (NavAb) (PDB code 3rvz)18 and that from the marine alphaproteobacterium HIMB114 (PDB code 4dxw),19 respectively. As for domain II, for which we could not build a reliable model, the crystal structure of NavAb (PDB code 3rvz) is tentatively shown as translucent gray ribbons and tubes. In (b), the cytoplasmic linker including the R1181Q mutation point between domain III and IV is just shown as dotted curves. The side chains of Leu312 and Val1020 are shown as van der Waals spheres colored red. DI–DIV, domain I–IV.
Trio-based WES was performed on two patients (patient 1 and 2) and patient-only WES on one patient (patient 3) using our cohort study of intellectual disability. De novo occurrence was confirmed for all the mutations by Sanger sequencing. The results of a TaqMan quantitative real-time PCR assay using the first-strand cDNA as a template of human fetal tissues and human adult tissues showed high expression in human brain (Supplementary Figure 1).
NALCN has four homologous repeat domains (I–IV) consisting of six transmembrane segments (S1–S6). The pairs of domains I and II, and domains II and IV are assembled closely to form NALCN channels.2, 15 Segments S5 and S6 of domains I–IV are predicted to compose a central pore-forming domain.2, 9 To evaluate the impact of the NALCN mutations, we constructed a model using the homology modeling Phyre2 server16 (as no experimental structure of human NALCN was available), and mapped the mutations on to the model. Residues p.Leu312 and p.Val1020 are predicted to be involved in the hydrophobic cores of the pore modules, responsible for gate opening and ion selectivity in domains I and III, respectively (Figure 1b). Thus, the p.L312V and p.V1020F mutations may affect the gating function of NALCN. Residue Arg1181 is located in the cytoplasmic loop connecting domain III with IV, suggesting that the p.R1181Q mutation is unlikely to affect an ion channel structure, but more likely affects interaction with other molecules. Previous studies have shown that the double knockout C. elegans mutant of nca-1 and nca-2 (nca-1(gk9); nca-2(gk5)) paralogous to human NALCN failed to sustain sinusoidal locomotion, which is a so called ‘fainter’ phenotype, and gain of functional allele (p.R43Q in nca-1) showed exaggerated body bends called ‘coiler’.10 A p.R1230Q nca-1 mutant, which is an analogous mutation of p.R1181Q in human NALCN, converted the fainter (nca-1(gk9); nca-2(gk5)) and wild type animals to coilers, indicating a dominant gain-of-function effect of this mutation.10 Again, including our patient, there have been three patients with intellectual disability, arthrogryposis and hypotonia all with an heterozygous mutation at Arg1181,9, 10 suggesting that this mutation results in improper NALCN function in both C. elegans and humans.
All the 17 missense mutations occurring de novo in NALCN were positioned in either S5 or S6 of domains I–III, as shown in Figure 2a. In contrast, recessive mutations (p.Y497Tfs*21,6 p.W1287L,6 and p.Q642X5) were unrelated to pore-forming domains (Figure 1a). The p.Y497Tfs*216 and p.Q642X5 are predicted to cause severe truncation of NALCN leading to loss of domain III and IV, suggesting that the function of the mutant protein would be severely impaired. Alternatively, mutant transcripts harboring p.Y497Tfs*216 and p.Q642X5 mutations may undergo nonsense-mediated mRNA decay in patient cells. Therefore it is very likely that both mutations would lead to the loss of function in NALCN. Functional analysis was not yet reported concerning the p.W1287L mutation, but considering autosomal recessive inheritance, the mutation might lead to the loss of function effect. Therefore, it is likely that NALCN missense mutations in S5 and S6 have dominant-negative effects and recessive mutations including a missense mutation located in outside of pore-forming domains would cause loss-of-function effects.
Clinical features of the hands and feet of three patients with NALCN mutations. Left (a) and right (b) hands showing abduction of the thumbs and ulnar deviation of the wrist of patient 1; right (c) and left (d) feet showing clubfoot and dorsal flexion of first toes of patient 1. Right (e) and left (f) hands of patient 2 showing camptodactyly; right foot (g) of patient 2 with dorsal flexion of the first toe. Right hand (h) of patient 3 showing mild ulnar deviation. A full color version of this figure is available at the Journal of Human Genetics journal online.
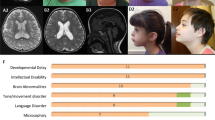
The clinical features of the three patients were carefully assessed. As shown in Figures 2a–d, patient 1 had small hands with distal digital hypoplasia, abducted thumbs, ulnar deviation of her wrist and bilateral clubfeet with dorsal flexion of her first toes. Patient 2 also had relatively small hands, camptodactyly, overlapping fingers, ulnar deviation of her wrist and dorsal flexion of her first toes (Figures 2 e–g). Patient 3 showed camptodactyly, overlapping fingers (Figure 2h) but her first toe was not as flexed compared with the toes of patients 1 and 2. All three patients were diagnosed as intellectual disability and had global developmental delay, some facial and limb dysmorphic features, and hypotonia (Table 1). Dorsal flexion of the first toes was observed in patient 1 and patient 2, and seems characteristic. Brain MRI of patient 1 showed cerebellar atrophy and loss of white matter; enlarged subarachnoid spaces were seen in patient 3 (data not shown). It is likely that NALCN mutations lead to a variety of brain abnormalities. The three patients in this study and the 15 previously reported patients had some common clinical features including intellectual disability, arthrogryposis, hypotonia, camptodactyly and ulnar deviation of the wrists.9, 10 Facial dysmorphisms and severe psychomotor impairment are commonly observed in patients with both dominant and recessive mutations (indicated in Table 1), and could be the main clinical features in patients with NALCN abnormalities. Hypotonia was observed in all eight recessive patients, and 11/16 dominant patients. Three dominant patients died before the age of five years. Joint contractions were observed in 14/17 dominant patients, and 2/2 recessive INAD patients (a 21-year-old female and an 18-year-old male) in whom contractures of extremities gradually developed.5 Three patients with p.R1181Q had severe psychomotor impairment, facial dysmorphisms and arthrogryposis. One of them had ataxic gait but could walk short distances independently, although another (patient 2) has never walked. Other clinical features vary. Since the number of recessive patients are limited and full information regarding symptomatic ages are not indicated, it is difficult to conclude whether the dominant (or recessive) patients show more severe phenotypes.
Although neurodevelopmental impairment and hypotonia are overlapping features of dominant and recessive mutations in the same gene, distal arthrogryposis, camptodactyly, and ulnar deviation are only observed in those with dominant mutations. More information on the clinical features associated with NALCN mutations is needed for better clinical practice.
References
Lu, B., Su, Y., Das, S., Liu, J., Xia, J. & Ren, D. The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129, 371–383 (2007).
Stephens, R. F., Guan, W., Zhorov, B. S. & Spafford, J. D. Selectivity filters and cysteine-rich extracellular loops in voltage-gated sodium, calcium, and NALCN channels. Front. Physiol. 6, 153 (2015).
Senatore, A. & Spafford, J. D. A uniquely adaptable pore is consistent with NALCN being an ion sensor. Channels 7, 60–68 (2013).
Ren, D. Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 72, 899–911 (2011).
Koroglu, C., Seven, M. & Tolun, A. Recessive truncating NALCN mutation in infantile neuroaxonal dystrophy with facial dysmorphism. J. Med. Genet. 50, 515–520 (2013).
Al-Sayed, M. D., Al-Zaidan, H., Albakheet, A., Hakami, H., Kenana, R., Al-Yafee, Y. et al. Mutations in NALCN cause an autosomal-recessive syndrome with severe hypotonia, speech impairment, and cognitive delay. Am. J. Hum. Genet. 93, 721–726 (2013).
Mok, K. Y., Schneider, S. A., Trabzuni, D., Stamelou, M., Edwards, M., Kasperaviciute, D. et al. Genomewide association study in cervical dystonia demonstrates possible association with sodium leak channel. Mov. Disord. 29, 245–251 (2014).
Cochet-Bissuel, M., Lory, P. & Monteil, A. The sodium leak channel, NALCN, in health and disease. Front Cell Neurosci. 8, 132 (2014).
Chong, J. X., McMillin, M. J., Shively, K. M., Beck, A. E., Marvin, C. T., Armenteros, J. R. et al. De novo mutations in NALCN cause a syndrome characterized by congenital contractures of the limbs and face, hypotonia, and developmental delay. Am. J. Hum. Genet. 96, 462–473 (2015).
Aoyagi, K., Rossignol, E., Hamdan, F. F., Mulcahy, B., Xie, L., Nagamatsu, S. et al. A gain-of-function mutation in NALCN in a Child with intellectual disability, ataxia, and arthrogryposis. Hum. Mutat. 36, 753–757 (2015).
Fukai, R., Hiraki, Y., Yofune, H., Tsurusaki, Y., Nakashima, M., Saitsu, H. et al. A case of autism spectrum disorder arising from a de novo missense mutation in POGZ. J. Hum. Genet. 60, 277–279 (2015).
Ng, P. C. & Henikoff, S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 31, 3812–3814 (2003).
Adzhubei, I. A., Schmidt, S., Peshkin, L., Ramensky, V. E., Gerasimova, A., Bork, P. et al. A method and server for predicting damaging missense mutations. Nat. Methods 7, 248–249 (2010).
Schwarz, J. M., Rodelsperger, C., Schuelke, M. & Seelow, D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat. Methods 7, 575–576 (2010).
Strong, M., Chandy, K. G. & Gutman, G. A. Molecular evolution of voltage-sensitive ion channel genes: on the origins of electrical excitability. Mol. Biol. Evol. 10, 221–242 (1993).
Kelley, L. A. & Sternberg, M. J. Protein structure prediction on the Web: a case study using the Phyre server. Nat. Protoc. 4, 363–371 (2009).
Kowal, J., Chami, M., Baumgartner, P., Arheit, M., Chiu, P. L., Rangl, M. et al. Ligand-induced structural changes in the cyclic nucleotide-modulated potassium channel MloK1. Nat. Commun. 5, 3106 (2014).
Payandeh, J., Scheuer, T., Zheng, N. & Catterall, W. A. The crystal structure of a voltage-gated sodium channel. Nature 475, 353–358 (2011).
Zhang, X., Ren, W., DeCaen, P., Yan, C., Tao, X., Tang, L. et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature 486, 130–134 (2012).
Acknowledgements
We thank the patients and their families for participating in this work. We were supported in part by a grant for Research on Measures for Intractable Diseases, a grant for Comprehensive Research on Disability Health and Welfare, the Strategic Research Program for Brain Science (SRPBS) and a grant for Initiative on Rare and Undiagnosed Diseases in Pediatrics (IRUD-P) from the Japan Agency for Medical Research and Development (AMED); a Grant-in-Aid for Scientific Research on Innovative Areas (Transcription Cycle) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); Grants-in-Aid for Scientific Research (A, B, and C), and challenging Exploratory Research from the Japan Society for the Promotion of Science (JSPS); the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Japan Science and Technology Agency (JST); the Takeda Science Foundation; the Yokohama Foundation for Advancement of Medical Science; and the Hayashi Memorial Foundation for Female Natural Scientists.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Supplementary information
Rights and permissions
About this article
Cite this article
Fukai, R., Saitsu, H., Okamoto, N. et al. De novo missense mutations in NALCN cause developmental and intellectual impairment with hypotonia. J Hum Genet 61, 451–455 (2016). https://doi.org/10.1038/jhg.2015.163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2015.163
This article is cited by
-
Structure of voltage-modulated sodium-selective NALCN-FAM155A channel complex
Nature Communications (2020)
-
Structure of the human sodium leak channel NALCN in complex with FAM155A
Nature Communications (2020)
-
Functional expression of CLIFAHDD and IHPRF pathogenic variants of the NALCN channel in neuronal cells reveals both gain- and loss-of-function properties
Scientific Reports (2019)
-
Methylation determines the extracellular calcium sensitivity of the leak channel NALCN in hippocampal dentate granule cells
Experimental & Molecular Medicine (2019)