Abstract
We examined a Korean family with complex phenotypes characterized by intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness. Since we did not find any abnormality using several conventional genetic tests for detection of chromosomal aberrations, gene copy number variations and mitochondrial gene mutations, we aimed to identify disease-causing genetic alteration(s) in this family. We conducted whole-exome sequencing (WES) in this family. After filtering the WES data, we compared five exome sequences of two affected siblings, one unaffected sibling and the unaffected parents, and we determined the allele frequency of the identified variants in an Asian population. Finally, we selected one candidate variant pair which is unique in the patients and corresponds to an autosomal recessive genetic model. The two affected siblings had the same compound heterozygous variation in the NEB gene encoding nebulin, which was composed of two different missense variants: c.2603T>C (p.L868P) in exon 27 and c.21340C>T (p.R7114W) in exon 143. We confirmed these variations by Sanger sequencing. On the basis of the fundamental role of nebulin in the brain and skeletal muscles, we concluded that this compound heterozygous NEB variation may be a sound candidate for the disease-causing mutation in this family. Since the patients are characterized by generalized muscle weakness together with neurodevelopmental phenotypes, it is suggested that NEB mutations could manifest more diverse phenotypes than those previously described.
Similar content being viewed by others
Introduction
We examined a Korean patient characterized by intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness. Her older brother had a similar clinical phenotype, but her parents and older sister were not affected. Copy number variants have been reported to be common in patients with multiple neurodevelopmental disorders, such as a combination of intellectual disability and epilepsy.1 Many genes have been reported to be responsible for a variety of congenital myopathies.2 To identify the causative genetic alterations in the affected siblings, we first performed several genetic tests, including G-band karyotyping for detection of chromosomal aberrations, array comparative genomic hybridization analysis for detection of copy number variation, Southern blot analysis for detection of CGG expansion in the FMR1gene which is the causative gene for Fragile X syndrome, and sequencing analysis for detection of mutations in the mitochondrial DNA. However, we did not find any abnormalities with these mutation analyses.
Recently, whole-exome sequencing (WES) with a next-generation sequencer has been shown to be robust in identifying causative gene(s) in inherited disorders.3 WES in 264 patients with epileptic encephalopathy and in their parents by the Epi4K Consortium and Epilepsy Phenome/Genome Project found many novel gene mutations.4 Diagnostic exome sequencing analysis in persons with severe intellectual disability found a lot of de novo mutations.5 Therefore, we carried out WES in this family. Here, we report rare compound heterozygous NEB variants in Korean patients with intellectual disability, epilepsy and generalized muscle weakness. This is the first report that NEB mutations known to cause autosomal recessive nemaline myopathy may also be involved in neurodevelopmental phenotypes. Therefore, we have discussed the possible genotype–phenotype correlations, mainly focusing on conjectured nebulin function in neurons as an actin regulator.
Materials and methods
Subjects
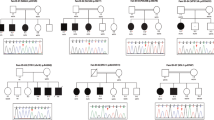
Two affected siblings (II-2 and II-3) visited the Department of Physical Medicine and Rehabilitation, Ajou University Hospital, Suwon, Republic of Korea, for their intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness (Figure 1a). To identify the causative genetic alterations in the affected siblings, we first performed several genetic tests, including G-band karyotyping for the detection of chromosomal aberrations, array comparative genomic hybridization analysis for the detection of copy number variation using the Roche NimbleGen CGX-3 135K Whole-Genome Array (Roche NimbleGen, Inc., Madison, WI, USA), Southern blot analysis for the detection of CGG expansion in the FMR1gene for Fragile X syndrome, and sequencing analysis for the detection of mutations in mitochondrial DNA. However, we did not find any abnormalities using any of these approaches. We collected peripheral blood samples from the five family members for WES and extracted genomic DNA using standard procedures, where the unaffected parents (I-1 and I-2) and unaffected older sister (II-1) were recruited as controls for the mutation analysis (Figure 1a). This study was approved by the Institutional Review Board Committee of the Ajou University Medical Center.
Pedigree of the family in this study and Sanger sequencing results of NEB variations. (a) Pedigree of the parents (I-1 and I-2), the two affected siblings (II-2 and II-3) and the one unaffected sibling (II-1) who underwent the quintet-based whole-exome sequencing. The letter ‘P’ indicates a proband of this family. (b) Sanger sequencing results of the variations in the NEB gene (c.2603T>C and c.21340C>T) in the parents (I-1 and I-2) and in the patients (II-2 and II-3). All chromatograms and sequences are the results of reverse-primer sequencing. The corresponding sequences are shown on top of each chromatogram.
Whole-exome sequencing with a next-generation sequencer
A pre-enrichment DNA library was constructed according to the Illumina TrueSeq DNA sample preparation guide (Illumina, Inc., San Diego, CA, USA). Exome enrichment was done using Illumina TrueSeq Exome Enrichment probes and streptavidin beads. The enriched exome library was loaded onto flow cells of an Illumina cBot for cluster generation. The flow cells with clusters of the enriched exome libraries were transferred to an Illumina HiSeq2000. High-throughput sequencing was then performed for each captured library to ensure that each sample met the desired average sequencing depth of 40-fold. A full description of the WES methods is included in the Supplementary Methods.
Sanger sequencing
DNA extracted from peripheral blood samples was amplified using PCR with primers to confirm the c.2603T>C in exon 27 (5′-CTACCCTTGGGCATCGTAAC-3′ and 5′-GGCATGTGTGATGTCTTTGC-3′) and the c.21340C>T in exon 143 (5′-GCCCCATCACAGTACCTGAC-3′ and 5′-TGGCCCTCTGGAGTGTTTAC-3′). The PCR product was sequenced on an ABI 3500xL DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
Results
Clinical description
A 15-year-old girl (II-3 in Figure 1a) came to the clinic with complex phenotypes of intellectual disability, epilepsy and generalized muscle weakness of early-childhood onset. She was born by normal vaginal delivery. Her birth weight was 3 500 g. Her development was apparently unremarkable until the age of 12 months. At the age of 13 months, she developed nonfebrile seizure episodes, which were not controlled by various antiseizure medicines. Along with intractable epilepsy, she showed progressive psychomotor regression and eventually became wheelchair-bound at the age of 13 years with no production of meaningful words. She did not show dysmorphic features and had a normal head circumference (53.0 cm, 5th to 25th percentile). She showed generalized muscle weakness, where the manual muscle test revealed grade 3/3 of the upper extremities and grade 1/1 in the lower extremities, respectively (Table 1). She had a ‘skin and bones’ appearance in all four extremities due to severe muscle atrophy. The patient had severe contracture of multiple joints including bilateral hips, knees, ankles, shoulders, elbows, wrists and interphalangeal joints along with scoliosis (Figure 2). She did not exhibit any signs of spasticity, dystonia or rigidity and her muscle enzymes were within the normal range.
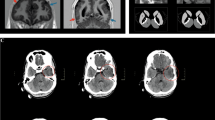
Radiographic findings of the patient (II-3). Magnetic resonance imaging of the brain shows normal findings in the top panel, where sagittal T1-, axial T1- and T2-weighted images are given. The plain X-rays of the spine and knees reveals scoliosis and 130°/150° bilateral flexion contracture of the knees in the bottom panels.
Muscle biopsy is a good method to determine if myopathy is present. However, a muscle biopsy could not be done because the patient refused the procedure. Instead, a needle electromyography test was done in both the tibialis anterior and gastrocnemius muscles of the proband. Although she showed little volitional muscle contraction due to scanty muscle tissue with fibrosis, a few short duration, low amplitude polyphasic motor units were identified as well as a few abnormal spontaneous activities with positive sharp waves and fibrillations, indicating that she has myopathy. While she was able to open and close both eyes with no difficulty, she had considerable difficulty with mastication and swallowing. A videofluoroscopic swallowing study revealed significant delays in both the oral and pharyngeal phase of swallowing, showing fluid aspiration without developing a cough reflex. However, tube feeding has been refused and she has been on oral feeding with pureed diet.
A psychological evaluation using the Korean Wechsler Adult Intelligence Scale and the Korean Vineland Social Maturity Scale showed profound intellectual disability, with a full-scale intellectual quotient of <30, a verbal intellectual quotient of <30, a performance intellectual quotient of <30, and a social quotient of 16.1 (Table 1). She was completely dependent with respect to performance of the activities of living, with a score of zero on the modified Barthel index. Magnetic resonance imaging of the brain showed normal findings (Figure 2). The electroencephalogram study showed generalized epileptiform discharges, with an irregular and discontinuous background rhythm.
Her older brother (II-2 in Figure 1a) had similar clinical phenotypes (Table 1). He showed apparently normal development until the age of 6 months and had become wheelchair-bound by the age of 10 years. Her parents and older sister did not have any myopathic or neurodevelopmental problems. We could not detect any genetic alterations in the affected two siblings by conventional genetic tests.
Whole-exome DNA sequence analysis
We conducted WES analysis of the family members as described above. After data filtering, we compared the five exome sequences from the two affected siblings, the unaffected sibling and their unaffected parents. We excluded non-disease-causing common variants, and extracted exonic and splicing variants that could explain the cause of the disease in the patients according to autosomal dominant or recessive genetic models, or genomic imprinting mutations as described in the Supplementary Methods. As a result, a total of seven compound heterozygous variant pairs of five genes (PRAMEF2, NEB, IBSP, PRKAG2 and MLH3) were identified as candidate variants corresponding to the autosomal recessive genetic model (Supplementary Table S1). Next, we determined the allele frequency of the candidate variants in an Asian population on the basis of the data from the 1000 Genomes Project (http://www.1000genomes.org/), and selected those with an allele frequency of 1% or less. Finally, only one compound heterozygous NEB variant pair, which is unique in the patients, was found. The patients had two different NEB missense variants, c.2603T>C (p.L868P) in exon 27 and c.21340C>T (p.R7114W) in exon 143, but the unaffected parents and older sister had only one NEB variant (either c.2603T>C or c.21340C>T; Table 2). We confirmed these variations in the family members by Sanger sequencing (Figure 1b).
In silico prediction of functional effects for the missense variants
As these two variants have not been reported in the Human Gene Mutation Database (http://www.hgmd.cf.ac.uk), we conducted in silico prediction of the functional effect of these variations at the amino-acid level using Polymorphism Phenotyping v2 (PolyPhen 2) and Sorting Intolerant from Tolerant predicting methods. The PolyPhen 2 results predicted that both variants are probably damaging, and the Sorting Intolerant from Tolerant results predicted that the p.R7114W variant is intolerant (Table 2). Furthermore, the amino-acid residues in the variation sites are well conserved in other species (Supplementary Figure S1). These in silico prediction results indicate that both of these NEB variants may have a deleterious effect on the protein’s function (Table 2).
Discussion
We identified the NEB variant of alternatively spliced exon 143 in addition to the constitutive exon 27. Clinically the affected subjects are characterized by intellectual disability and epilepsy in addition to congenital myopathy of early-childhood onset. A giant filamentous protein nebulin encoded by NEB gene is one of actin regulators like dystrophin, utrophin, α-actinin, β-spectrin and so on.6,7 Nebulin is expressed abundantly in skeletal muscle as a component of the cytoskeletal matrix.8 Alternative splicing results in numerous nebulin isoforms differing in their C-terminal regions.9 Although the isoforms of nebulin are expressed in other organs in addition to skeletal muscle, particularly in the brain,10 the biological function of nebulin remains unknown in the brain. Mutations in NEB cause autosomal recessive congenital myopathies such as nemaline myopathy 2 (NEM2, OMIM #256030), distal myopathy and core-rod myopathy.11,12 Nemaline myopathy patients having NEB gene mutations present a wide spectrum of morphological features.13
The severe generalized muscle weakness concurrent with contracture of multiple joints seen in our patients is compatible with typical clinical findings of autosomal recessive congenital myopathy caused by NEB mutations. However, to the best of our knowledge, there have been no reports describing any signs of dysfunction of the CNS such as intellectual disability and/or epilepsy in patients with NEB mutations. We concluded that the compound heterozygous NEB variation detected herein might be a disease-causing mutation for the following reasons: (1) our patients showed severe early-childhood-onset generalized muscle weakness; (2) nebulin is reported to be expressed in the brain, as well as in the skeletal muscle; (3) nebulin is an actin regulator that seems to have a role in neurons, as well as in skeletal muscle; (4) the two NEB variants identified in our patients are very rare in the general population; and (5) the two NEB variants are predicted in silico to have deleterious effects on the nebulin protein.
The isoforms of nebulin are expressed in other organs in addition to skeletal muscle, particularly in the brain.10 An immunohistochemical study reported that nebulin is expressed in the cytoplasm of pyramidal neurons and in subcortical endothelial cells in the brain.10 Therefore, nebulin seems to have a role as an actin regulator in neurons of the human brain as it does in skeletal muscle. Actin is involved in many essential processes in eukaryotic cells, including muscle cells and neurons. Through actin polymerization, actin works as a universal force provider, generating the pressure that biological processes use.14 Since abnormal morphological changes of neuronal dendrites and spines are reported to be associated with intellectual disability, changes in the cytoskeletal organization of dendrites and spines are likely to affect the structure and function of developing and mature synapses.15 Therefore, abnormalities of actin-binding proteins could cause dysfunction of the actin cytoskeleton. It seems that synaptic excitability and synaptic plasticity could be altered by a dysfunctional actin cytoskeleton caused by NEB mutations. Similar phenomenon is observed in Duchenne muscular dystrophy (OMIM #310200), which is a fatal, recessive, X-linked muscular disease caused by Dystrophin gene mutation.16 Dystrophin is also one of actin regulators like nebulin and is expressed in both muscle fibers and nonmuscular organs including the brain.17 Deficiency of the brain-specific isoforms of dystrophin protein such as Dp71 and Dp140 is known to be associated with the presence of intellectual disability and seizure in Duchenne muscular dystrophy.18,19 Therefore, it is not surprising that our patients had intellectual disability and epilepsy, as these are associated with a biallelic mutation of the actin regulator-encoding NEB gene.
Our patients had compound heterozygous missense variation in exons 27 and 143. Of the 183 exons in the NEB gene, exons 63–66, 82–105, 143–144 and 166–177 are key regions where alternative splicing occurs.20 Alternative splicing in one of the four regions is likely to be quite elaborate in a developmental stage-specific, organ-specific or muscle type-specific way. Since exons 143–144 are known to be mutually exclusive in alternative splicing, these two exons are never found within the same transcript.20 A mouse study reported that the transcripts expressing Neb exon 127, which corresponds to human NEB exon 143, were more prominent in muscles of young mice.21 Transcripts expressing either exons 143 or 144 are found in both the muscles and brain.10 They encode a portion of amino acids from the super-repeat 21 in muscles and the linker repeat 1 in the brain, which is close to the carboxy (C)-terminal region. Maintaining physiological Z-disk widths and myofibrillar connectivity is known as one of the functions of nebulin, where the C-terminal region of nebulin interacts with desmin close to the Z-disk.22,23 This is also possibly relevant for the exon 143 functions of NEB. Amino acid sequences encoded by exons 143 or 144 differ in both charge and hydrophobicity and the amino-acid sequence encoded by exon 143 shows complete homology between mouse, rat and human.21 In the human fetal brain, only exon 144-containing transcripts were detected, whereas exon 143- or 144-containing transcripts were found in adult brains.10 The human fetal muscle expresses transcripts containing only exon 143, whereas the adult skeletal muscle and heart muscle express transcripts containing exon 143 or 144.20 This suggests that exon 143 might harbor a regulatory function utilized during muscle maturation. However, there has been no report on mutation involving exons 143 and 144 of NEB in human, yet.11,20,21
In this context, we concluded that the identified compound heterozygous NEB variation may be a sound candidate for the disease-causing mutation in our patients characterized by intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness, suggesting NEB mutations could manifest more diverse phenotypes than those previously described. Because the affected family is quite small and there are no strong linkage data in this family, however, more complex non-monogenetic mechanisms involved in the identified NEB variations need to be considered. In addition, functional studies are required to elucidate the possible mechanisms underlying the CNS dysfunction in these rare compound heterozygous NEB variants.
References
Mullen SA, Carvill GL, Bellows S, Bayly MA, Trucks H, Lal D et al. Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology 81, 1507–1514 (2013).
Sewry CA Pathological defects in congenital myopathies. J. Muscle Res. Cell Motil. 29, 231–238 (2008).
Ku CS, Naidoo N & Pawitan Y Revisiting Mendelian disorders through exome sequencing. Hum. Genet. 129, 351–370 (2011).
Epi4K Consortium ; Epilepsy Phenome/Genome Project, Allen AS, Berkovic SF, Cossette P, Delanty N et al. De novo mutations in epileptic encephalopathies. Nature 2013; 501: 217–221.
de Ligt J, Willemsen MH, van Bon BW, Kleefstra T, Yntema HG, Kroes T et al. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 367, 1921–1929 (2012).
Siripala AD & Welch MD SnapShot: actin regulators II. Cell 128, 1014 (2007).
Siripala AD & Welch MD SnapShot: actin regulators I. Cell 128, 626 (2007).
Labeit S, Ottenheijm CA & Granzier H Nebulin, a major player in muscle health and disease. FASEB J. 25, 822–829 (2011).
Labeit S & Kolmerer B The complete primary structure of human nebulin and its correlation to muscle structure. J. Mol. Biol. 248, 308–315 (1995).
Laitila J, Hanif M, Paetau A, Hujanen S, Keto J, Somervuo P et al. Expression of multiple nebulin isoforms in human skeletal muscle and brain. Muscle Nerve 46, 730–737 (2012).
Lehtokari VL, Pelin K, Sandbacka M, Ranta S, Donner K, Muntoni F et al. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum. Mutat. 27, 946–956 (2006).
Scoto M, Cullup T, Cirak S, Yau S, Manzur AY, Feng L et al. Nebulin (NEB) mutations in a childhood onset distal myopathy with rods and cores uncovered by next generation sequencing. Eur. J. Hum. Genet. 21, 1249–1252 (2013).
Malfatti E, Lehtokari VL, Bohm J, De Winter JM, Schaffer U, Estournet B et al. Muscle histopathology in nebulin-related nemaline myopathy: ultrastrastructural findings correlated to disease severity and genotype. Acta Neuropathol. Commun. 2, 44 (2014).
Xue B & Robinson RC Guardians of the actin monomer. Eur. J. Cell Biol. 92, 316–332 (2013).
Conde C & Caceres A Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319–332 (2009).
Hoffman EP, Brown RH Jr & Kunkel LM Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Sekiguchi M The role of dystrophin in the central nervous system: a mini review. Acta Myol. 24, 93–97 (2005).
Moizard MP, Toutain A, Fournier D, Berret F, Raynaud M, Billard C et al. Severe cognitive impairment in DMD obvious clinical indication for Dp71 isoform point mutation screening. Eur. J. Hum. Genet. 8, 552–556 (2000).
Lidov HG, Selig S & Kunkel LM Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Hum. Mol. Genet. 4, 329–335 (1995).
Donner K, Sandbacka M, Lehtokari VL, Wallgren-Pettersson C & Pelin K Complete genomic structure of the human nebulin gene and identification of alternatively spliced transcripts. Eur. J. Hum. Genet. 12, 744–751 (2004).
Donner K, Nowak KJ, Aro M, Pelin K & Wallgren-Pettersson C Developmental and muscle-type-specific expression of mouse nebulin exons 127 and 128. Genomics 88, 489–495 (2006).
Bang ML, Gregorio C & Labeit S Molecular dissection of the interaction of desmin with the C-terminal region of nebulin. J. Struct. Biol. 137, 119–127 (2002).
Tonino P, Pappas CT, Hudson BD, Labeit S, Gregorio CC & Granzier H Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle. J. Cell Sci. 123, 384–391 (2010).
Acknowledgements
This work was supported by a 2012 grant from the Department of Medical Sciences, The Graduate School, Ajou University (M2012C046000028) and a National Research Foundation of Korea grant (NRF) funded by the Korean government (2014 001483).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Jin, HS., Lee, JB., Kim, K. et al. Identification of the rare compound heterozygous variants in the NEB gene in a Korean family with intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness. J Hum Genet 59, 643–647 (2014). https://doi.org/10.1038/jhg.2014.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2014.87
This article is cited by
-
Whole-exome sequencing in Tricho-rhino-phalangeal syndrome (TRPS) type I in a Korean family
Genes & Genomics (2017)
-
A reply to a commentary on identification of the rare compound heterozygous variants in the NEB gene in a Korean family with intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness
Journal of Human Genetics (2015)
-
A commentary on identification of the rare compound heterozygous variants in the NEB gene in a Korean family with intellectual disability, epilepsy and early-childhood-onset generalized muscle weakness
Journal of Human Genetics (2015)