Abstract
Charcot–Marie–Tooth disease (CMT), the most common hereditary neuropathy, has been classified into two types, demyelinating and axonal types. We previously analyzed the genes causing dominant demyelinating CMT in 227 Japanese patients to identify the genetic background, but could not find any mutations in 110 patients. To investigate the frequency of patients with autosomal recessive demyelinating CMT (CMT4) mutations, we analyzed the coding sequence of known causative genes of CMT4 in 103 demyelinating CMT patients, excluding seven patients owing to lack of specimens. We found one patient with a GDAP1 mutation, one patient with an MTMR2 mutation, two patients with SH3TC2/KIAA1985 mutations and three patients with FGD4 mutations. Twelve patients, including five previously detected patients with PRX mutations, were diagnosed as CMT4, accounting for 5.5% of demyelinating CMT. In the patient with GDAP1 mutation, only one mutation inherited from his mother was detected by genomic sequencing. Analysis by reverse transcription polymerase chain reaction using messenger RNA (mRNA) from the patient’s leukocytes revealed the absence of transcription from the allele inherited from his father, suggesting the existence of one more mutation leading to a lack or destabilization of mRNA. Most patients carrying CMT4 gene mutations present with early-onset and slowly progressive symptoms, which may be associated with the function of mutants. We could not identify the disease-causing gene in 96 patients (about 45%). Further studies including studies with next-generation sequencers will be required to identify the causative gene in Japanese CMT.
Similar content being viewed by others
Introduction
Charcot–Marie–Tooth disease (CMT) is the most common hereditary neuromuscular disorder, with a prevalence estimated at up to 5–40 individuals in every 100 000. Motor and sensory peripheral nerves are affected, and clinical features comprise slowly progressive distal muscle weakness and atrophy and glove-stocking-type sensory disturbance.1, 2 CMT has been traditionally classified into two types by electrophysiological studies: demyelinating and axonal types. Recently, a group showing intermediate median motor nerve conduction velocities (MCV) and overlapping demyelinating and axonal CMT has been referred to as intermediate CMT.2 More than 30 genes have been identified as CMT disease-causing genes.1, 2, 3
To identify the genetic background of Japanese CMT, we analyzed the disease-causing genes in about 350 patients; however, we could not identify the causative genes in about 50% of demyelinating CMT and 80% of axonal CMT. In the demyelinating type, a low prevalence of PMP22 duplication is a feature of Japanese patients, which likely leads to the high frequency of unknown cause patients.4 The Japanese patients carrying PMP22 duplication seem to have mild symptoms due to genetic and or epigenetic-modifying factors and do not use medical services, reflecting a low prevalence.4
In the present study, we analyzed the genes causing autosomal recessive demyelinating CMT (CMT4) to further clarify the genetic background of Japanese patients.
Materials and methods
The Ethics Committee of Yamagata University School of Medicine approved this study. Genomic DNA and RNA were prepared from peripheral blood specimens after written informed consent was obtained from the patients and their families.
Patients
Two hundred twenty-seven patients with demyelinating CMT were recruited in this study. Of the 227 patients, 93 patients with autosomal dominant CMT (CMT1),4, 5, 6, 7, 8, 9, 10, 11, 12 19 patients with X-linked inheritance (CMTX),8, 13, 14 5 patients with PRX mutations4, 15, 16 and 7 patients who lacked DNA samples were excluded (Figure 1). The remaining 103 patients (32 with and 71 patients without family history) were unrelated, and 14 patients were from consanguineous families.
Screening by genomic DNA
Genomic DNA was extracted from peripheral blood using a standard method. The genes targeted in this study were CMT4-causing genes: GDAP1 (ganglioside-induced differentiation-associated protein 1),17 MTMR2 (myotubularin-related protein 2),18, 19 SBF2/MTMR13 (SET binding factor 2/myotubularin-related protein 13),20 SH3TC2/KIAA1985 (SH3 domain and tetratricopeptide repeats 2),21 NDRG1 (N-Myc downstream-regulated gene 1),22 FGD4 (FYVE, RhoGEF and PH domain containing 4),23, 24 and FIG4 (FIG4 homolog, SAC1 lipid phosphatase domain containing).25 We amplified all coding regions and their exon–intron boundaries using a set of PCR primers designed by Primer 3 software based on the genomic information (details available upon request) or a set of primers as described in previous reports: GDAP1,26 MTMR2,27 SH3TC2/KIAA1985,28 NDRG122 and FGD4.24 Sequencing of PCR products was directly determined using a dye-terminator reaction (BigDye Terminator v. 1.1, Applied Biosystems, Foster City, CA, USA) with an ABI 3500 × l automated genetic analyzer (Applied Biosystems). The number of nucleotides was based on the published online protein and messenger RNA sequences of the respective genes (www.ncbi.nlm.nih.gov).
Analysis by reverse transcription polymerase chain reaction
To ascertain that the mutations of GDAP1 and FGD4 bring about abnormal splicing, we performed reverse transcription polymerase chain reaction (RT-PCR) analysis.29, 30 Total RNA was extracted from the leukocytes of each patient who carried the mutations of FGD4 at the splice site (patients 6 and 7) and who had a mutation in GDAP1 in one allele (patient 1), with ISOGEN-LS (Nippon Gene Co., Tokyo, Japan). Subsequently, messenger RNA (mRNA) was prepared using an Oligotex-dT30 Super mRNA purification kit (Takara Bio, Otsu, Japan) and was reverse transcribed into cDNA with random primers using a TaKaRa RNA PCR kit (AMV) ver. 3.0 (Takara Bio). cDNAs were then amplified using each set of PCR primers; primers for GDAP1 were: F, 5′-CAGTGTGGGAGGGAGAAGTC-3′; R, 5′-ATGCTTGGATGAGCTGCC-3′ (1178-bp fragment from wild-type mRNA); those for exons 6–8 of FGD4 were: F, 5′-GCAAACTGTTGGAAGAAGCA-3′; R, 5′-AGGGAATCAGGAGGCAATTT-3′ (412-bp fragment from wild-type mRNA);24 and those for exons 7–9 of FGD4 were: F, 5′-TTGATAATGCAATGGAATTGG-3′; R, 5′-AGAATGGCTTGCTGCTGTAG-3′ (254-bp fragment from wild-type mRNA). PCR products of FGD4 were directly sequenced and those of GDAP1 were subcloned into a TA vector and these sequences were determined. Relative amounts of each GDAP1 transcript were estimated by the number of colonies.
Results
We analyzed the CMT4-causing genes in 103 patients from 220 patients with demyelinating CMT who had no mutations in CMT1 or CMTX1-causing genes (Figure 1). Seven cases were found to carry CMT4-causing gene mutations: one case with a GDAP1 mutation, one case with an MTMR2 mutation, two cases with SH3TC2/KIAA1985 mutations and three cases with FGD4 mutations. Table 1 shows the type of mutation and clinical information of each patient. We detected 12 cases with mutation in CMT4 genes in all, including five cases carrying PRX mutations that were previously reported,4, 15, 16 accounting for 5.5% of all patients with demyelinating CMT (Table 2).
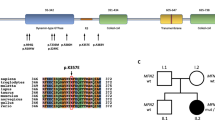
Patient 1 carried a heterozygous GDAP1 c.571C>T (p.R191*) mutation in exon 4. His mother was also a heterozygote of the mutation but was not affected. To find another mutation on the allele inherited from his father, RT-PCR analysis was performed. We extracted mRNA from leukocytes of patient 1, his mother and a healthy control. We amplified a 1178-bp fragment from c.−63 to c.*38 of GDAP1 and found two major bands on agarose gel electrophoresis in the specimens from the control, mother and patient 1 (Figure 2). The sequence of each band was determined after subcloning and all transcripts were classified into three sizes: ∼1200, 1050 and 950 bp (Figure 3). The wild allele produces three types of transcripts, 1, 2 and 3, by alternative splicing, as shown in control specimens. His mother had three types of transcripts from wild and mutant alleles, and two other alternatively spliced transcripts (4 and 5), likely derived from the mutant allele, which lacked exon 4 due to c.571C>T in exon 4. Patient 1 had four transcripts identical to the transcripts from the mutant allele of his mother; however, patient 1 lacked transcript 3 from the mutant allele and had transcript 6 transcribed from the mutant allele. All transcripts except transcript 1 from the wild allele are predicted to make premature terminal codons 5′ to the last 50 nucleotides of the penultimate exon and would be degraded by nonsense-mediated decay.31 However, some amount of transcript 2 from a c.571C>T mutant allele was detected, suggesting that small amounts of a truncated peptide were produced at least in the leukocytes of patient 1 and his mother. By RT-PCR analysis, we could not find any mRNA from the allele inherited from his father in the patient, indicating that patient 1 likely had another mutant allele inherited from his father leading to a lack or destabilization of mRNA.
Splicing variations of GDAP1 in patient 1. Transcript 1 (1178 bp) is a full length of messenger RNA (mRNA). Transcript 2 (mRNA lacks the 145 bp of the 3′ part of exon 2), transcript 3 (mRNA lacks an entire exon 2) and transcript 6 (mRNA lacks an entire exon 3) are registered as wild-type variations. Black boxes represent exon 4 carrying the c.571C>T mutation inherited from his mother. RT-PCR products of GDAP1 were subcloned into a TA vector and these sequences were determined. Relative amounts of each transcript of GDAP1 were estimated by the numbers of colonies and are described in parentheses.
Patient 2 was a homozygote of the MTMR2 c.1882_1885delAGAG mutation. This mutation causes a frame shift and was predicted to produce a prolonged protein (p.R628Pfs*18). Her mother was a heterozygote for the mutation and no specimen from her father was available. A nerve biopsy revealed myelin outfoldings.
We found SH3TC2/KIAA1985 mutations in two patients; patient 3 was a homozygote of the c.3379C>T (p.R1127W) mutation and patient 4 was a homozygote of the c.3511C>T (p.R1171C) mutation.
FGD4 mutations were detected in three patients: patient 5 was a homozygote of the c.1888_1892delAAAGG (p.K630Nfs*4) mutation, patient 6 was a compound heterozygote of the c.837−2A>G and c.1132+1G>A mutations and patient 7 was a homozygote carrying the c.837-1G>A mutation. The c.837−2A>G, c.1132+1G>A and c.837−1G>A mutations are predicted to cause splicing abnormalities. RT-PCR analysis confirmed that those mutations lead to frame shift, and they were predicted to produce the truncated proteins, p.W279*fs, p.Y355Ifs*2 and p.E280Kfs*23, respectively (Figures 4 and 5). Patients 5 and 6 had similarly affected siblings carrying the same mutations. Patient 7 showed myelin outfoldings in the nerve biopsy specimens.
All mutations except the GDAP1 c.571C>T (p.R191*) mutation32 were novel and were not detected in 100 healthy controls.
Discussion
We performed a broad genetic screening in 220 patients with demyelinating CMT and detected 93 CMT1 patients (42.3%), including 53 patients with PMP22 duplication,4, 5, 6, 7, 8, 9, 10, 11, 12 19 CMTX patients8, 13, 14 (8.6%) and 12 CMT4 patients4, 15, 16 (5.5%) (Table 2). As for the frequency of autosomal recessive CMT, there are few studies in the literature.3 Sapora et al.3 analyzed 381 patients with demyelinating CMT and found 7 CMT4 patients (1.8%). In the Mediterranean Sea area and the Middle East, it is reported that autosomal recessive CMT is more frequent than in North America and Europe because of a high percentage of consanguineous marriages.33, 34 We detected 12 patients with mutations in CMT4 genes and nine of them were homozygotes for the mutation. The percentage of CMT4 may reflect the percentage of consanguineous marriages (3.88%) in Japan: lower than that (20% to over 50%) in the Mediterranean Sea domain and the Middle East and higher than that (<1.0%) in the USA.35, 36, 37 There are still 96 patients (43.6%) with unknown causes.
GDAP1 is a tail-anchored protein of the outer mitochondrial membrane and it regulates the dynamics of the mitochondrial network by inducing mitochondrial fragmentation.17, 38 Mutations in GDAP1 are responsible for the most frequent recessive form of demyelinating and axonal CMT. We found a heterozygous p.R191* mutation in patient 1, but did not find any other mutation. The p.R191* mutation was reported as one of the mutations detected in a compound heterozygote,32 indicating that patient 1 should have had one more mutation on an allele inherited from his father. RT-PCR analysis revealed no transcript from the allele from his father. We determined the sequence of the promoter region and poly-A additional signals (data not shown), but could not find any mutations. The patient likely has an unknown mutation in an allele inherited from his father leading to a lack or destabilization of mRNA. The patient developed hypotonia and a foot deformity within the first year of life.
MTMR2 encodes a protein that belongs to the myotubularin family, which is characterized by the presence of a phosphatase domain, and may play a significant role in neural membrane recycling, membrane trafficking and endo- and exocytic processes.18, 19 We found a homozygous c.1882_1885delAGAG mutation in patient 2. The c.1882_1885delAGAG mutation is located in the last exon, and is predicted to escape nonsense-mediated decay and produce a prolonged peptide, p.R628Pfs*18. Most patients carrying MTMR2 mutations present symptoms in early infancy and have a subsequent severe course;18, 39 however, patient 2 presented at 13 years of age with mild clinical symptoms. The p.R628Pfs*18 mutant protein may be partly functional and therefore only cause mild symptoms. Myelin outfoldings and redundant myelin loops are characteristic findings in the mutation of MTMR2, MTMR13 and FGD4.2, 18, 23 Patient 2 revealed these characteristic pictures on nerve biopsy (data not shown).
SH3TC2/KIAA1985 plays a significant role in membrane trafficking and is required for proper myelination and integrity of the node of Ranvier.21 We found homozygous missense SH3TC2/KIAA1985 mutations in two patients: a c.3379C>T (p.R1127W) mutation in patient 3 and a c.3511C>T (p.R1171C) mutation in patient 4. The specimens from both families were not available and segregation analysis could not be performed. However, c.3379C>T and c.3511C>T mutations were not detected in 100 healthy controls. R1127 and R1171 are conservative amino-acid residues, and scores from PolyPhen-2 software analysis (http://genetics.bwh.harvard.edu/pph2) to predict the functional effect of p.R1127W and p.R1171C were 1, indicating that SH3TC2/KIAA1985 variants were probably damaging and disease-causing mutations. The clinical phenotype associated with SH3TC2/KIAA1985 mutations is variable and is mostly characterized by early onset and frequent complication of scoliosis.40, 41, 42 Baets et al.40 reported SH3TC2/KIAA1985 was the most commonly mutated recessive gene in CMT, presenting with symptoms within the first year of life in Western countries and the Middle East. The onset age of our cases was infancy, but they did not have scoliosis. Patient 3 showed progressive gait disturbance from 4 years of age. Patient 4 had gait disturbance in childhood and showed slowly progressive symptoms and developed hearing loss and respiratory distress in his seventies.
Frabin encoded by FGD4 likely has a key role in proliferation, polarization, survival of Schwann cells and myelination processes.23, 24, 29 We found FGD4 mutations in three cases: a homozygous c.1888_1892delAAAGG (p.K630Nfs*4) mutation in patient 5, compound heterozygous c.837−2A>G (p.W279*fs) and c.1132+1G>A (p.Y355Ifs*2) mutations in patient 6, and a homozygous c.837−1G>A (p.E280Kfs*23) mutation in patient 7. All four mutations are predicted to make premature terminal codons 5′ to the last 50 nucleotides of the penultimate exon and should be degraded by nonsense-mediated decay.31 In the RT-PCR analysis experiment, transcripts from c.837−2A>G and c.1132+1G>A mutant alleles in leukocytes from patient 6 were detected in one-third of transcripts from the wild allele in control leukocytes. Transcripts from the c.837−1G>A mutant allele in leukocytes from patient 7 were also decreased to two-thirds of that from wild alleles in control leukocytes. Therefore, these mutations are predicted to produce some amount of truncated peptides (Figures 4 and 5). Quantitative and qualitative abnormalities in FGD4 may be associated with the pathogenesis in the patients. Patient 7 showed myelin outfolding on nerve biopsy. Three patients showed childhood onset and moderately severe and slowly progressive symptoms as in other reported cases.43
We systematically analyzed 220 Japanese patients with demyelinating CMT (7 cases were excluded due to a lack of specimens) and found only 12 patients carrying autosomal recessive demyelinating CMT gene mutations. The autosomal recessive demyelinating CMT mutations likely behave in a loss-of-function manner. Therefore, we cannot exclude the possibility that the patients may have microdeletion of the causative genes, which would not be detected from ordinal analysis using PCR. Our study indicated that RT-PCR analysis is useful to confirm splicing abnormalities by mutant alleles and for the screening gene mutations, as shown in patient 1. To identify the causative gene, analysis using next-generation sequencers44 and analysis of gene-expressing tissues derived from induced pluripotent stem cells would be powerful tools.
Most patients with CMT4 gene mutations present with early-onset and slowly progressive symptoms, in contrast to the early-onset and rapidly progressive symptoms in patients carrying the dominant gene mutation.15, 45 These differences may be associated with the type of mutant, which acts in a loss- or gain-of-function manner.
References
Banchs, I., Casasnovas, C., Albertí, A., De Jorge, L., Povedano, M., Montero, J. et al. Diagnosis of Charcot-Marie-Tooth disease. J. Biomed. Biotechnol. 2009, 985415 (2009).
Pareyson, D. & Marchesi, C. Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol. 8, 654–667 (2009).
Saporta, A. S., Sottile, S. L., Miller, L. J., Feely, S. M., Siskind, C. E. & Shy, M. E. Charcot-Marie-Tooth disease subtypes and genetic testing strategies. Ann. Neurol. 69, 22–33 (2011).
Abe, A., Numakura, C., Kijima, K., Hayashi, M., Hashimoto, T. & Hayasaka, K. Molecular diagnosis and clinical onset of Charcot-Marie-Tooth disease in Japan. J. Hum. Genet. 56, 364–368 (2011).
Abe, A., Numakura, C., Saito, K., Koide, H., Oka, N., Honma, A. et al. Neurofilament light chain polypeptide gene mutations in Charcot-Marie-Tooth disease: nonsense mutation probably causes a recessive phenotype. J. Hum. Genet. 54, 94–97 (2009).
Ikegami, T., Ikeda, H., Chance, P. F., Kiyosawa, H., Yamamoto, M., Sobue, G. et al. Facilitated diagnosis of CMT1A duplication in chromosome 17p11.2-12: analysis with a CMT1A-REP repeat probe and photostimulated luminescence imaging. Hum. Mutat. 9, 563–566 (1997).
Ikegami, T., Ikeda, H., Aoyama, M., Matsuki, T., Imota, T., Fukuuchi, Y. et al. Novel mutations of the peripheral myelin protein 22 gene in two pedigrees with Dejerine-Sottas disease. Hum. Genet. 102, 294–298 (1998).
Numakura, C., Lin, C., Ikegami, T., Guldberg, P. & Hayasaka, K. Molecular analysis in Japanese patients with Charcot-Marie-Tooth disease: DGGE analysis for PMP22, MPZ, and Cx32/GJB1 mutations. Hum. Mutat. 20, 392–398 (2002).
Abe, A., Nakamura, K., Kato, M., Numakura, C., Honma, T., Seiwa, C. et al. Compound heterozygous PMP22 deletion mutations causing severe Charcot-Marie-Tooth disease type 1. J. Hum. Genet. 55, 771–773 (2010).
Ikegami, T., Ikeda, H., Mitsui, T., Hayasaka, K. & Ishii, S. Novel mutation of the myelin Po gene in a pedigree with Charcot-Marie-Tooth disease type 1B. Am. J. Med. Genet. 71, 246–248 (1997).
Ikegami, T., Nicholson, G., Ikeda, H., Ishida, A., Johnston, H., Wise, G. et al. De novo mutation of the myelin Po gene in Déjérine-Sottas disease (hereditary motor and sensory neuropathy type III): two amino acid insertion after Asp 118. Hum. Mutat. 1, 103–105 (1998).
Numakura, C., Shirahata, E., Yamashita, S., Kanai, M., Kijima, K., Matsuki, T. et al. Screening of the early growth response 2 gene in Japanese patients with Charcot-Marie-Tooth disease type 1. J. Neurol. Sci. 210, 61–64 (2003).
Lin, C., Numakura, C., Ikegami, T., Shizuka, M., Shoji, M., Nicholson, G. et al. Deletion and nonsense mutations of the connexin 32 gene associated with Charcot-Marie-Tooth disease. Tohoku J. Exp. Med. 188, 239–244 (1999).
Ikegami, T., Lin, C., Kato, M., Itoh, A., Nonaka, I., Kurimura, M. et al. Four novel mutations of the connexin 32 gene in four Japanese families with Charcot-Marie-Tooth disease type 1. Am. J. Med. Genet. 80, 352–355 (1998).
Kijima, K., Numakura, C., Shirahata, E., Sawaishi, Y., Shimohata, M., Igarashi, S. et al. Periaxin mutation causes early-onset but slow-progressive Charcot-Marie-Tooth disease. J. Hum. Genet. 49, 376–379 (2004).
Otagiri, T., Sugai, K., Kijima, K., Arai, H., Sawaishi, Y., Shimohata, M. et al. Periaxin mutation in Japanese patients with Charcot-Marie-Tooth disease. J. Hum. Genet. 51, 625–628 (2006).
Niemann, A., Ruegg, M., La Padula, V., Schenone, A. & Suter, U. Ganglioside- induced differentiation associated protein 1 is a regulator of the mitochondrial network: new implications for Charcot-Marie-Tooth disease. J. Cell Biol. 170, 1067–1078 (2005).
Berger, P., Bonneick, S., Willi, S., Wymann, M. & Suter, U. Loss of phosphatase activity in myotubularin-related protein 2 is associated with Charcot-Marie-Tooth disease type 4B1. Hum. Mol. Genet. 11, 1569–1579 (2002).
Begley, M. J., Taylor, G. S., Brock, M. A., Ghosh, P., Woods, V. L. & Dixon, J. E. Molecular basis for substrate recognition by MTMR2, a myotubularin family phosphoinositide phosphatase. Proc. Natl Acad. Sci. USA 103, 927–932 (2006).
Senderek, J., Bergmann, C., Weber, S., Ketelsen, U. P., Schorle, H., Rudnik-Schöneborn, S. et al. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum. Mol. Genet. 12, 349–356 (2003).
Arnaud, E., Zenker, J., De Preux Charles, A. S., Stendel, C., Roos, A., Medard, J. J. et al. SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system. Proc. Natl Acad. Sci. USA 106, 17528–17533 (2009).
Kalaydjieva, L., Gresham, D., Gooding, R., Heather, L., Baas, F., De Jonge, R. et al. N-myc downstream-regulated gene 1 is mutated in hereditary motor and sensory neuropathy-Lom. Am. J. Hum. Genet. 67, 47–58 (2000).
Stendel, C., Roos, A., Deconinck, T., Pereira, J., Castagner, F., Niemann, A. et al. Peripheral nerve demyelination caused by a mutant Rho GTPase guanine nucleotide exchange factor, frabin/FGD4. Am. J. Hum. Genet. 81, 158–164 (2007).
Delague, V., Jacquier, A., Hamadouche, T., Poitelon, Y., Baudot, C., Boccaccio, I. et al. Mutations in FGD4 encoding the Rho GDP/GTP exchange factor FRABIN cause autosomal recessive Charcot-Marie-Tooth type 4H. Am. J. Hum. Genet. 81, 1–16 (2007).
Chow, C. Y., Zhang, Y., Dowling, J. J., Jin, N., Adamska, M., Shiga, K. et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature 448, 68–72 (2007).
Cuesta, A., Pedrola, L., Sevilla, T., García-Planells, J., Chumillas, M. J., Mayordomo, F. et al. The gene encoding ganglioside-induced differentiation-associated protein 1 is mutated in axonal Charcot-Marie-Tooth type 4A disease. Nat. Genet. 30, 22–25 (2002).
Bolino, A., Muglia, M., Conforti, F. L., LeGuern, E., Salih, M. A., Georgiou, D. M. et al. Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat. Genet. 25, 17–19 (2000).
Senderek, J., Bergmann, C., Stendel, C., Kirfel, J., Verpoorten, N., De Jonghe, P. et al. Mutations in a gene encoding a novel SH3/TPR domain protein cause autosomal recessive Charcot-Marie-Tooth type 4C neuropathy. Am. J. Hum. Genet. 73, 1106–1119 (2003).
Fabrizi, G. M., Taioli, F., Cavallaro, T., Ferrari, S., Bertolasi, L., Casarotto, M. et al. Further evidence that mutations in FGD4/frabin cause Charcot-Marie-Tooth disease type 4H. Neurology 72, 1160–1164 (2009).
Houlden, H., Hammans, S., Katifi, H. & Reilly, M. M. A novel Frabin (FGD4) nonsense mutation p.R275X associated with phenotypic variability in CMT4H. Neurology 72, 617–620 (2009).
Holbrook, J. A., Neu-Yilik, G., Hentze, M. W. & Kulozik, A. E. Nonsense-mediated decay approaches the clinic. Nat. Genet. 36, 801–808 (2004).
Baránková, L., Vyhnálková, E., Züchner, S., Mazanec, R., Sakmaryová, I., Vondrácek, P. et al. GDAP1 mutations in Czech families with early-onset CMT. Neuromuscul. Disord. 17, 482–489 (2007).
Dubourg, O., Azzedine, H., Verny, C., Durosier, G., Birouk, N., Gouider, R. et al. Autosomal-recessive forms of demyelinating Charcot-Marie-Tooth disease. Neuromol. Med. 8, 75–86 (2006).
Patzkó, A. & Shy, M. E. Update on Charcot-Marie-Tooth disease. Curr. Neurol. Neurosci. Rep. 11, 78–88 (2011).
Imaizumi, Y. A recent survey of consanguineous marriages in Japan. Clin. Genet. 30, 230–233 (1986).
Bittles, A. H. Consanguinity and its relevance to clinical genetics. Clin. Genet. 60, 89–98 (2001).
Lebel, R. R. Consanguinity studies in Wisconsin I: secular trends in consanguineous marriage, 1843-1981. Am. J. Med. Genet. 15, 543–560 (1983).
Noack, R., Frede, S., Albrecht, P., Henke, N., Pfeiffer, A., Knoll, K. et al. Charcot-Marie-Tooth disease CMT4A: GDAP1 increases cellular glutathione and the mitochondrial membrane potential. Hum. Mol. Genet. 21, 150–162 (2012).
Gambardella, A., Bono, F., Muglia, M., Valentino, P. & Quattrone, A. Autosomal recessive hereditary motor and sensory neuropathy with focally folded myelin sheaths (CMT4B). Ann. N. Y. Acad. Sci. 883, 47–55 (1999).
Baets, J., Deconinck, T., De Vriendt, E., Zimoń, M., Yperzeele, L., Van Hoorenbeeck, K. et al. Genetic spectrum of hereditary neuropathies with onset in the first year of life. Brain 134, 2664–2676 (2011).
Laššuthová, P., Mazanec, R., Vondráček, P., Sišková, D., Haberlová, J., Sabová, J. et al. High frequency of SH3TC2 mutations in Czech HMSN I patients. Clin. Genet. 80, 334–345 (2011).
Houlden, H., Laura, M., Ginsberg, L., Jungbluth, H., Robb, S. A., Blake, J. et al. The phenotype of Charcot-Marie-Tooth disease type 4C due to SH3TC2 mutations and possible predisposition to an inflammatory neuropathy. Neuromuscul. Disord. 19, 264–269 (2009).
Baudot, C., Esteve, C., Castro, C., Poitelon, Y., Mas, C., Hamadouche, T. et al. Two novel missense mutations in FGD4/FRABIN cause Charcot-Marie-Tooth type 4H (CMT4H). J. Peripher. Nerv. Syst. 17, 141–146 (2012).
Lupski, J. R., Reid, J. G., Gonzaga-Jauregui, C., Rio Deiros, D., Chen, D. C., Nazareth, L. et al. Whole-genome sequencing in a patient with Charcot-Marie-Tooth neuropathy. N. Engl. J. Med. 362, 1181–1191 (2010).
Hayasaka, K., Himoro, M., Sawaishi, Y., Nanao, K., Takahashi, T., Takada, G. et al. De novo mutation of the myelin P0 gene in Dejerine-Sottas disease (hereditary motor and sensory neuropathy type III). Nat. Genet. 5, 266–268 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, M., Abe, A., Murakami, T. et al. Molecular analysis of the genes causing recessive demyelinating Charcot–Marie–Tooth disease in Japan. J Hum Genet 58, 273–278 (2013). https://doi.org/10.1038/jhg.2013.15
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2013.15
Keywords
This article is cited by
-
Clinical genetics of Charcot–Marie–Tooth disease
Journal of Human Genetics (2023)
-
Molecular characterization of Turkish patients with demyelinating Charcot-Marie-Tooth disease
neurogenetics (2022)
-
Clinical and mutational spectrum of Japanese patients with recessive variants in SH3TC2
Journal of Human Genetics (2018)