Abstract
Large-scale deletions of human mitochondrial DNA (mtDNA) are a common cause of mitochondrial diseases. In order to prevent and treat these mitochondrial diseases, it is important and necessary to understand the mechanisms behind the generation of these deletions. Generally, there exist three kinds of large-scale deletions: deletions almost occur within two direct repeats with identical sequences (class I deletions), deletions are flanked by imperfect repeats (class II deletions) and by no direct repeats (class III deletions). Two major hypotheses are suggested to generate these deletions: replication for class I/II deletions through slipped mispairing between two repeats, and repair mainly for class II/III deletions mediated by mtDNA double-strand breaks. It seems possible that these two mechanisms work together as a powerful and complementary system to compensate for their defects in the generation of all these deletions, not respectively.
Similar content being viewed by others
Mitochondria and mitochondrial DNA
Mitochondria are ubiquitous organelles, which convert food into energy in the form of ATP through oxidative phosphorylation. Mitochondrial oxidative phosphorylation is composed of complex I, II, III and IV, and complex V, that is, the ATP synthase. In brief, electrons from the carbohydrates and fats are transferred to complex I or complex II, then flow through ubiquinone (CoQ) to complex III and finally went through cytochrome (Cyt c) to complex IV to generate H2O. The energy released in this way is used to pump out protons through complex I, III and IV to create an electrochemical gradient. These high-capacitance protons flow back to complex V with concomitant production of ATP, which is used as a source of chemical energy supply for the process of life.1
Human mitochondrial DNA (mtDNA), as the only ex-nuclear genome, is a small (16 569 bp), economical and double-stranded closed circular molecule.2 The outer G-rich strand, called heavy strand (H strand) can encode the 12S and 16S ribosomal RNAs (rRNAs), 14 transfer RNAs (tRNAs) and 12 polypeptides, the other inner C-rich strand named light strand (L strand) can only encode eight tRNAs and one polypeptide (ND6). In total, it contains 37 genes encoding 13 peptides for the oxidative phosphorylation apparatus, 7 for subunits of complex I, 1 for subunits of complex III, 3 for subunits of complex IV and 2 for subunits of complex V, as well as 22 tRNAs and two rRNAs.1 D-loop region is the only one non-coding region in mtDNA, which has 1211 base pairs with some important and essential region, including the H-strand origin of replication (OH), some transcription factor binding sites, L strand promoters and conserved sequence blocks.3 Therefore, it has an important role in replication, transcription, translation and evolution of mtDNA. The L-strand replication origin (OL) is two-thirds the circumference around the mtDNA from the OH in the D-loop. The two origins of replication (OH and OL) divide the mtDNA into two arcs: the minor arc that includes D-loop, the 12S rRNA, 16S rRNA, ND1, ND2 and some tRNAs and the major arc that includes the other genes of mtDNA (Figure 1).
A human mitochondrial DNA map showing deletion frequency of every gene and relationship between some mtDNA deletions and mitochondrial diseases. The number in every gene represents the deletion frequency of this gene according to MITOMAP. Arcs represent the deleted regions and arrows indicate the relationship. Deletion frequency is calculated by the number of one gene lost to the total number of each gene lost in all deletions. Single letters inside are abbreviations of the 22 tRNAs. Abbreviations: KSS, Kearns–Sayre syndrome; PEO, progressive external ophthalmoplegia; OH, the H strand origin of replication; OL, the L strand origin of replication. A full color version of this figure is available at the Journal of Human Genetics journal online.
Mitochondria, the only semiautonomous organelle in human cells, have their own separate system for mtDNA replication, transcription, translation and repair of damaged mtDNA. Mitochondrial dysfunction, including that associated with mtDNA mutation, has been increasingly recognized as an important factor in a wide range of human diseases.4, 5
Mitochondrial DNA mutation in human diseases
Lack of protective histone, poor repair system and high frequent exposure to reactive oxygen species, generated as a toxic by-product from mitochondrial oxidative phosphorylation, exposes mtDNA at high risk to damage.6, 7, 8 Point mutations (including mini insertions and deletions) and major rearrangement mutations (deletions and insertions) are common in human mitochondrial diseases and aging people.3, 9 Most point mutations of mtDNA are homoplasmic,10, 11 but pathogenic mutations are usually heteroplasmic.12 Compared with point mutations, mtDNA large-scale deletions occurred less frequently, nevertheless they contribute much more to mitochondrial diseases, cancer and aging. Different deletions have been demonstrated with relation to aging,13, 14 various types of cancer15, 16, 17, 18, 19 and mitochondrial diseases, such as Kearns–Sayre syndrome,20, 21, 22 myopathies,23, 24 progressive external ophthalmoplegia,22, 25, 26 diabetes,27 deafness27 and so on (Figure 1). The 4977 bp common deletion and the ∼7.4 kb deletions are the top two deletions most associated with mitochondrial diseases,3 because they can both influence the mitochondrial protein synthesis due to the loss of at least one tRNA gene and one coding region.28 mtDNA deletions have been considered as the first pathogenic mutations in human mtDNA.29
Deletions have been reported in different tissues from the same patient, and demonstrated in various types of human cancer or some mitochondrial diseases at different deletion level.13, 15, 30, 31, 32, 33 Although all large-scale mtDNA deletions are heteroplasmic and sometimes the deletion level is low, as long as the deleted mtDNA is present, it is prone to expansion quickly because of its replicative advantage.33 Meanwhile, being exposed continuously to the endogenous reactive oxygen species, the level of mutant mtDNA would accumulate until a biochemical and physiological defect is expressed.34, 35, 36 In order to prevent these patients from mitochondrial diseases and improve the current treatment, we need to enhance our understanding of the cause and pathogenesis of these mitochondrial diseases.
Distribution and classification of mitochondrial DNA deletions
Analyzed by Southern blotting, PCR and DNA direct sequencing, mtDNA deletions have been detected and their position at 5′ and 3′ breakpoints have been calculated.37 Most of the deletion breakpoints occur within two directly repeated sequences, which are thought to cause most large-scale mtDNA deletions.37, 38 Among the 263 pathogenic deletions, the 5′ and 3′ ends had a uniform distribution mostly within the major arc.38 According to MITOMAP, we concluded all large-scale deletions and also found that the deleted regions frequently locate in the major arc, especially the ND4 and ND5 gene (Figure 1). Furthermore, these deletions almost occur within perfect repeats (68%) (class I deletions), whereas 12% deletions are flanked by imperfect repeats (class II deletions) and 20% deletions are flanked by no direct repeats (class III deletions).3
mtDNA 4977 bp deletion has been frequently reported within the category of class I deletions. The two 13 bp perfect repeats (ACCTCCCTCACCA) have been found at mtDNA nucleotide positions 8470–8482 bp (in the ATPase8 gene) and 13447–13459 bp (in the ND5 gene) surrounding this deletion breakpoint. One repeat is retained, whereas the other is removed. This rearrangement mutation removes two complex V subunits, one complex IV subunit, four complex I subunits and five intervening tRNAs. The deleted 5 kb subgenomic fragment with no origins of replication locates concordantly in the center or at the hotspot of the distribution of deletions in the major arc.
At the direct repeat level, it was suggested that large-scale deletions occurred either by slipped mispairing or recombination, whereas different mechanisms may have caused the generation of deletions several years ago.23 And now, through further researches, we have developed a deeper understanding of their occurrence and could provide additional perspective for prevention of mitochondrial diseases associated with mtDNA large-scale deletions.
The generation of mtDNA large-scale deletions
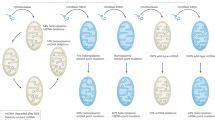
There has not been a united conclusion on how these deletions are generated. At least two major hypotheses are thought to be the cause of these formations (1) mtDNA replication20, 24, 37, 38 and (2) repair of damaged mtDNA.39, 40 Similarly, the two hypotheses are based on the fact that slipped mispairing or misannealing mediates the formation of human mtDNA deletions.
Replication-generated mitochondrial DNA deletions
Mitochondria foremost duplicates its mtDNA before it starts the division.41 mtDNA has two origins of replication, the OH and the OL. This characteristic is similar to bacteria and plasmids, but differs from human nuclear DNA. Partly unlike nuclear DNA replication, there are two main ways of mtDNA replication: a strand-asynchronous, asymmetric mode as proposed by Clayton et al.42, 43 and a synchronous, coupled leading- and lagging-strand synthesis mode as suggested by Holt et al.44 (Figure 2)
Slipped mispairing replication models behind the generation of mitochondrial DNA (mtDNA) deletions. Asynchronous replication (above) can form a single strand to give the two repeats a chance to mispair to generate mtDNA deletions, whereas synchronous replication (low) seem not have the capacity to make these.
A strand-asynchronous, asymmetric replication of mtDNA is a slow process beginning at the OH in the D-loop. The nascent H strand progresses abidingly at two-thirds the circumference around the L strand till the OL is exposed, then the nascent L strand begins to synthesize in the opposite direction. In this stage, most of the parent H strand (the major arc) is a single strand, which is thought to be vulnerable, to being out of control of the mtDNA replication systems. Because of replication error, large-scale deletions are considered to be generated via slipped mispairing between the two inter-strand repeats.20, 40, 44 If the 3′-repeat (taking the L strand to be the reference) of the parent H strand pairs with the downstream 5′-repeat of the L strand, a single-strand loop is then generated. Once a strand breaks and degradation takes place, a new deleted parent H strand will be ligated, subsequently be taken as a template for the synthesis of the daughter L strand from the OL. The two nascent strands replicate persistently as a circle around their separated templates, a new wild-type mtDNA and a deleted mtDNA will be synthesized.20, 40 (Figure 2).
Currently, most researchers consider that replication is the possible mechanism behind the deletions. Besides this theory, there are some strong reasons that make us to believe that the generation of mtDNA deletions is related to the mtDNA replication error.
-
1)
A high proportion of the reported mtDNA deletions occurred within the major arc of mtDNA between the two origins of replication. A few deletions would remove the OL, but the OH will be mostly conserved.3, 34, 45, 46 These features of mtDNA deletions are consistent with the hypotheses that replication generates deletions. An entire mtDNA replication starts from the OH and finishes at the OH. The nascent L strand does not begin to synthesize until the OL is exposed when the nascent H strand synthesis has processed about two-thirds away from the OH. The single parent H strand between the two origins (the major arc) emerges and gives a chance for the two repeats downstream from the OH to come together to generate mtDNA deletion via slipped mispairing replication.40
-
2)
A special phenomenon observed in the 4977 bp common deletion was consistent with the postulate that mtDNA deletions occurred during the slipped mispairing replication. This deletion occurs between two perfect repeats, located at 8470–8482 and 13447–13459 bp, respectively. Conventionally, the nucleotide sequence from nt8483 to nt13446 should be deleted. However, Degoul et al.23 found that the adenosine at nt13446 near the 3′-repeat close to the OH was retained. At the replication direction and the retained adenosine lever, once again, this implies that the slipped mispairing replication generates mtDNA deletions.
-
3)
In the process of replication, how can two repeats that are sometimes situated far from each other around the molecule come together and mispair to form a single strand loop? We have already known that mtDNA is not straight, but almost curved, during replication. Schon et al.37 found two AT-rich regions located near the common deletion breakpoint. Rocher et al.47 suggested that long regions of homopyrimidine/homopurine or pyrimidine content are present, surrounding the deletion breakpoints. Solano et al.29 identified that, sometimes, there were some palindrome sequences present within or near the deletion breakpoints. They all considered that these characterizations of mtDNA may make it easy to alter its conformation and then bend. Once mtDNA bends to a degree, there would be a chance for the two repeats to mispair to form a single-strand loop. After the single-strand loop breaks and degrades, the H strand ligates to become a template for synthesis of the deleted mtDNA. Furthermore, special binding proteins can induce and stabilize bends in the front of the human mtDNA L strand replication origin in the process of DNA replication.48 And recently, DNA bending in TATA-binding proteins/TATA complexes has been proposed in nuclear DNA by Wu et al.49 Therefore, by a combination of the several natures of mtDNA and Wu's experimental results, it is plausible to say that mtDNA could bend in the process of replication to induce the two intramolecular repeats of the different strands to form a single-strand loop, which is a prerequisite for the generation of mtDNA large-scale deletions.
There have been many evidences leading us to believe that the slipped mispairing replication as the major mechanism behind mtDNA large-scale deletions generation. However, there are some equivocal doubts about it. For example, these abovementioned evidences are dependent on a situation that a single strand must be exposed during the process of mtDNA replication. To make this possible, only the strand-asynchronous replication can create this specific situation. When it comes to a synchronous, coupled leading- and lagging-strand synthesis mode of replication, these evidences will not be valid40 (Figure 2). Holt et al.44, 50, 51 and Brown et al.52 had different opinions about the mechanisms of mtDNA replication, but they both believed the strand-asynchronous, asymmetric replication occurred in mammalian mitochondria through detecting and analyzing the existence of replication intermediates with the help of two-dimensional agarose gel electrophoresis and atomic force microscopy. The two major modes of mtDNA replication can be found simultaneously in mammalian cells. These findings indicate that the strand-asynchronous mode of replication mediate the generation of mtDNA deletions, and the two modes or even only one accumulate these deletions. Furthermore, during the strand-asynchronous replication, the generation of class III deletions still remains to be unanswered, as there were no repeats at the deletion breakpoints, neither does the slipped mispairing seem to work.
Repair-generated mitochondrial DNA deletions
Recently, mtDNA deletions are considered likely to be generated during the repair of double-strand breaks (DSBs) of mtDNA.40, 53, 54 In mammalian cells, mtDNA DSBs can be caused by ionizing radiation or by some endogenous factors including replication pausing.55, 56 In other words, DSBs can occur momentarily, thus repair becomes very important and necessary to maintain mtDNA integrity. There are two orthodox pathways to repair a DSB: homologous recombination and nonhomologous end-joining (NHEJ). These two repair partners differ in their requirements of direct repeats to pair at the junction.55, 56, 57, 58, 59, 60
During the homologous recombination repair pathway, the ends of the DSBs are susceptible to be resected completely by 3′ → 5′ exonucleases, allowing the homologous repeats to pair, so that the two free single-strand tails are exposed to be degraded. After the single-strand annealed absolutely, a new intact mtDNA with deletion is produced (Figure 3). Furthermore, DSBs of mtDNA seem likely to promote deletions without repeats, which implies that NHEJ also has a role in repairing this mtDNA damage,53, 54 although NHEJ repair has not been identified in neuronal mitochondria54, 61 (Figure 3).
There are also some experiments supporting this hypotheses that deleted mtDNA may be generated during the repair of damaged mtDNA with DSBs. They established animal models with DSBs through resecting the mtDNA by restriction endonuclease. Krishnan et al.40 found most of new deletions ligated between the restriction endonuclease sites and the D-loop or between two restriction endonuclease sites, but they did not explain clearly about which types of deletions occurred more frequently. In addition, other findings showed that DSBs-induced deletions contained no or small direct repeats, especially class II/III deletions.53, 54
However, there are also some concerns about this hypothesis. For instance, the mitochondrial DNA polymerase, POLG, has a well-characterized 3′ → 5′ exonuclease activity to mediate ligation of mtDNA DSBs.40 Furthermore, POLG seems not to have the capacity to degrade a large portion of mtDNA. Possibly, there are other uncharacterized exonucleases taking place in the generation of deletions.54 It seems that mtDNA deletions primarily occurred during NHEJ repair model rather than during homologous recombination model, as a small portion of mtDNA may need to be degraded during NHEJ. It is consistent with Moraes's finding that DSBs mainly mediates the formation of class II/III deletions.53, 54 Furthermore, all class I, class II and class III deletions can occur in the situation in which the same DSBs restricted by endonuclease pair with each other.53, 54 However, it seems to be a fact that single mtDNA deletions are common in sporadic mitochondrial diseases including Kearns–Sayre syndrome and chronic progressive external ophthalmoplegia.4, 40 Srivastava et al.53, 54 suggested that DSBs mainly mediated the formation of class II/III mtDNA deletions in mammals, whereas, in fact, class I deletions occur frequently in human cell.
Replication and repair, do they work independently or together?
There are three types of mtDNA deletions, 68% for class I deletions, 12% for class II deletions and 20% accounting for class III deletions. Replication remains valid to generate mtDNA deletions, which can only generate class I/II deletions, whereas at the DSBs level, it has been acknowledged that repair can generate all these deletions currently. In addition, class II/III deletions seem likely to be formed during repair.53, 54 On the basis of these phenomena, both of the two models have their flaws behind the generation of mtDNA deletions.54 Replication and repair can mainly generate only class I/II and class II/III deletions, respectively, (Figures 2 and 3). Taken the two models together, it may be a powerful and complementary system to generate deletions. However, it does not necessarily mean they always work simultaneously. Potentially, there is a selection between replication and repair mediating mtDNA large-scale deletions. Single large-scale deletions, a common cause of mitochondrial diseases, frequently occur at mild situation and mostly account for class I/II deletions. Here, replication is the dominant mechanism behind the formation of deletion. Ionizing radiation or some severe endogenous factors can arise mtDNA DSBs, DSBs-induced deletions mainly account for class II/III deletions, in which repair is the dominant or even independent mechanism leading to mtDNA deletions.
Conclusions
mtDNA mutations have been demonstrated to be related with many human mitochondrial diseases. Furthermore, in these mutations, deletions were the first pathogenic mutations.29 Now there is not a uniform mechanism leading to the occurrence of mtDNA deletions. Replication may cause class I/II deletions, and repair mainly cause class II/III deletions. However, it is a fact that class I, class II and class III deletions can coexist in a person or even in the same tissue.34 Boldly, replication and repair may work as a connected system to compensate for their defects in the generation of all these deletions.
Replication and repair would be the top two mechanisms behind the formation of mtDNA large-scale deletions. However, many concerns remain, and further work is required to test the two mechanisms or more. Improved understanding of the mechanisms involved in their generation will pave the way for prevention and treatment of mitochondrial diseases.
References
Shen, L., Fang, H., Chen, T., He, J., Zhang, M., Wei, X. et al. Evaluating mitochondrial DNA in cancer occurrence and development. Ann. NY Acad. Sci. 1201, 26–33 (2010).
Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J. et al. Sequence and organization of the human mitochondrial genome. Nature 290, 457–465 (1981).
MITOMAP: A Human Mitochondrial Genome Database.. http://www.mitomap.org (2010).
Taylor, R. W. & Turnbull, D. M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402 (2005).
Lu, J., Sharma, L. K. & Bai, Y. Implications of mitochondrial DNA mutations and mitochondrial dysfunction in tumorigenesis. Cell. Res. 19, 802–815 (2009).
Chen, J., Kadlubar, F. F. & Chen, J. Z. DNA supercoiling suppresses real-time PCR: a new approach to the quantification of mitochondrial DNA damage and repair. Nucleic Acids Res. 35, 1377–1388 (2007).
Richter, C., Park, J. W. & Ames, B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl Acad. Sci. USA 85, 6465–6467 (1988).
Ames, B. N., Shigenaga, M. K. & Hagen, T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl Acad. Sci. USA 90, 7915–7922 (1993).
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407 (2005).
Polyak, K., Li, Y., Zhu, H., Lengauer, C., Willson, J. K., Markowitz, S. D. et al. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat. Genet. 20, 291–293 (1998).
Fliss, M. S., Usadel, H., Caballero, O. L., Wu, L., Buta, M. R., Eleff, S. M. et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287, 2017–2019 (2000).
Park, J. S., Sharma, L. K., Li, H., Xiang, R., Holstein, D., Wu, J. et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum. Mol. Genet. 18, 1578–1589 (2009).
Meissner, C., Bruse, P., Mohamed, S. A., Schulz, A., Warnk, H., Storm, T. et al. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp. Gerontol. 43, 645–652 (2008).
Eshaghian, A., Vleugels, R. A., Canter, J. A., McDonald, M. A., Stasko, T. & Sligh, J. E. Mitochondrial DNA deletions serve as biomarkers of aging in the skin, but are typically absent in nonmelanoma skin cancers. J. Invest. Dermatol. 126, 336–344 (2006).
Kamalidehghan, B., Houshmand, M., Panahi, M. S., Abbaszadegan, M. R., Ismail, P. & Shiroudi, M. B. Tumoral cell mtDNA approximately 8.9 kb deletion is more common than other deletions in gastric cancer. Arch. Med. Res. 37, 848–853 (2006).
Tseng, L. M., Yin, P. H., Chi, C. W., Hsu, C. Y., Wu, C. W., Lee, L. M. et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in breast cancer. Genes Chromosomes Cancer 45, 629–638 (2006).
Wu, C. W., Yin, P. H., Hung, W. Y., Li, A. F., Li, S. H., Chi, C. W. et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes Chromosomes Cancer 44, 19–28 (2005).
Abnet, C. C., Huppi, K., Carrera, A., Armistead, D., McKenney, K., Hu, N. et al. Control region mutations and the ‘common deletion’ are frequent in the mitochondrial DNA of patients with esophageal squamous cell carcinoma. BMC Cancer 4, 30 (2004).
Chen, T., He, J., Shen, L., Fang, H., Nie, H., Jin, T. et al. The mitochondrial DNA 4977-bp deletion and its implication in copy number alteration in colorectal cancer. BMC Med. Genet. 12, 8 (2011).
Shoffner, J. M., Lott, M. T., Voljavec, A. S., Soueidan, S. A., Costigan, D. A. & Wallace, D. C. Spontaneous Kearns-Sayre/chronic external ophthalmoplegia plus syndrome associated with a mitochondrial DNA deletion: a slip-replication model and metabolic therapy. Proc. Natl Acad. Sci. USA 86, 7952–7956 (1989).
Zeviani, M., Moraes, C. T., DiMauro, S., Nakase, H., Bonilla, E., Schon, E. A. et al. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology 38, 1339–1346 (1988).
Moraes, C. T., DiMauro, S., Zeviani, M., Lombes, A., Shanske, S., Miranda, A. F. et al. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N. Engl. J. Med. 320, 1293–1299 (1989).
Degoul, F., Nelson, I., Amselem, S., Romero, N., Obermaier-Kusser, B., Ponsot, G. et al. Different mechanisms inferred from sequences of human mitochondrial DNA deletions in ocular myopathies. Nucleic Acids Res. 19, 493–496 (1991).
Manfredi, G., Vu, T., Bonilla, E., Schon, E. A., DiMauro, S., Arnaudo, E. et al. Association of myopathy with large-scale mitochondrial DNA duplications and deletions: which is pathogenic? Ann. Neurol. 42, 180–188 (1997).
Wong, L. J., Perng, C. L., Hsu, C. H., Bai, R. K., Schelley, S., Vladutiu, G. D. et al. Compensatory amplification of mtDNA in a patient with a novel deletion/duplication and high mutant load. J. Med. Genet. 40, e125 (2003).
Johns, D. R., Rutledge, S. L., Stine, O. C. & Hurko, O. Directly repeated sequences associated with pathogenic mitochondrial DNA deletions. Proc. Natl Acad. Sci. USA. 86, 8059–8062 (1989).
Ballinger, S. W., Shoffner, J. M., Hedaya, E. V., Trounce, I., Polak, M. A., Koontz, D. A. et al. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat. Genet. 1, 11–15 (1992).
Wallace, D. C. Mitochondrial DNA mutations and neuromuscular disease. Trends. Genet. 5, 9–13 (1989).
Solano, A., Gamez, J., Carod, F. J., Pineda, M., Playan, A., Lopez-Gallardo, E. et al. Characterisation of repeat and palindrome elements in patients harbouring single deletions of mitochondrial DNA. J. Med. Genet. 40, e86 (2003).
Rogounovitch, T., Saenko, V. & Yamashita, S. Mitochondrial DNA and human thyroid diseases. Endocr. J. 51, 265–277 (2004).
Zhang, C., Baumer, A., Mackay, I. R., Linnane, A. W. & Nagley, P. Unusual pattern of mitochondrial DNA deletions in skeletal muscle of an adult human with chronic fatigue syndrome. Hum. Mol. Genet. 4, 751–754 (1995).
Muqit, M. M., Larner, A. J., Sweeney, M. G., Sewry, C., Stinton, V. J., Davis, M. B. et al. Multiple mitochondrial DNA deletions in monozygotic twins with OPMD. J. Neurol. Neurosurg. Psychiatry. 79, 68–71 (2008).
Cortopassi, G. A., Shibata, D., Soong, N. W. & Arnheim, N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc. Natl Acad. Sci. USA 89, 7370–7374 (1992).
Bua, E., Johnson, J., Herbst, A., Delong, B., McKenzie, D., Salamat, S. et al. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am. J. Hum. Genet. 79, 469–480 (2006).
Ye, C., Shu, X. O., Wen, W., Pierce, L., Courtney, R., Gao, Y. T. et al. Quantitative analysis of mitochondrial DNA 4977-bp deletion in sporadic breast cancer and benign breast diseases. Breast Cancer Res. Treat. 108, 427–434 (2008).
Porteous, W. K., James, A. M., Sheard, P. W., Porteous, C. M., Packer, M. A., Hyslop, S. J. et al. Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur. J. Biochem. 257, 192–201 (1998).
Schon, E. A., Rizzuto, R., Moraes, C. T., Nakase, H., Zeviani, M. & DiMauro, S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science 244, 346–349 (1989).
Samuels, D. C., Schon, E. A. & Chinnery, P. F. Two direct repeats cause most human mtDNA deletions. Trends. Genet. 20, 393–398 (2004).
Albertini, A. M., Hofer, M., Calos, M. P. & Miller, J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell 29, 319–328 (1982).
Krishnan, K. J., Reeve, A. K., Samuels, D. C., Chinnery, P. F., Blackwood, J. K., Taylor, R. W. et al. What causes mitochondrial DNA deletions in human cells? Nat. Genet. 40, 275–279 (2008).
Gross, N. J., Getz, G. S. & Rabinowitz, M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J. Biol. Chem. 244, 1552–1562 (1969).
Robberson, D. L. & Clayton, D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase—derivatives: displacement replication on a covalently-closed circular template. Proc. Natl Acad. Sci. USA. 69, 3810–3814 (1972).
Clayton, D. A. Replication of animal mitochondrial DNA. Cell 28, 693–705 (1982).
Holt, I. J., Lorimer, H. E. & Jacobs, H. T. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell 100, 515–524 (2000).
Reeve, A. K., Krishnan, K. J., Elson, J. L., Morris, C. M., Bender, A., Lightowlers, R. N. et al. Nature of mitochondrial DNA deletions in substantia nigra neurons. Am. J. Hum. Genet. 82, 228–235 (2008).
Chang, D. D. & Clayton, D. A. Priming of human mitochondrial DNA replication occurs at the light-strand promoter. Proc. Natl Acad. Sci. USA 82, 351–355 (1985).
Rocher, C., Letellier, T., Copeland, W. C. & Lestienne, P. Base composition at mtDNA boundaries suggests a DNA triple helix model for human mitochondrial DNA large-scale rearrangements. Mol. Genet. Metab. 76, 123–132 (2002).
Welter, C., Dooley, S., Zang, K. D. & Blin, N. DNA curvature in front of the human mitochondrial L-strand replication origin with specific protein binding. Nucleic Acids Res. 17, 6077–6086 (1989).
Wu, J., Parkhurst, K. M., Powell, R. M., Brenowitz, M. & Parkhurst, L. J. DNA bends in TATA-binding protein-TATA complexes in solution are DNA sequence-dependent. J. Biol. Chem. 276, 14614–14622 (2001).
Bowmaker, M., Yang, M. Y., Yasukawa, T., Reyes, A., Jacobs, H. T., Huberman, J. A. et al. Mammalian mitochondrial DNA replicates bidirectionally from an initiation zone. J. Biol. Chem. 278, 50961–50969 (2003).
Yasukawa, T., Yang, M. Y., Jacobs, H. T. & Holt, I. J. A bidirectional origin of replication maps to the major noncoding region of human mitochondrial DNA. Mol. Cell. 18, 651–662 (2005).
Brown, T. A., Cecconi, C., Tkachuk, A. N., Bustamante, C. & Clayton, D. A. Replication of mitochondrial DNA occurs by strand displacement with alternative light-strand origins, not via a strand-coupled mechanism. Genes. Dev. 19, 2466–2476 (2005).
Srivastava, S. & Moraes, C. T. Double-strand breaks of mouse muscle mtDNA promote large deletions similar to multiple mtDNA deletions in humans. Hum. Mol. Genet. 14, 893–902 (2005).
Fukui, H. & Moraes, C. T. Mechanisms of formation and accumulation of mitochondrial DNA deletions in aging neurons. Hum. Mol. Genet. 18, 1028–1036 (2008).
Haber, J. E. Partners and pathwaysrepairing a double-strand break. Trends. Genet. 16, 259–264 (2000).
Richardson, C. & Jasin, M. Coupled homologous and nonhomologous repair of a double-strand break preserves genomic integrity in mammalian cells. Mol. Cell. Biol. 20, 9068–9075 (2000).
van Gent, D. C., Hoeijmakers, J. H. & Kanaar, R. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2, 196–206 (2001).
Rothkamm, K., Kruger, I., Thompson, L. H. & Lobrich, M. Pathways of DNA double-strand break repair during the mammalian cell cycle. Mol. Cell. Biol. 23, 5706–5715 (2003).
Takata, M., Sasaki, M. S., Sonoda, E., Morrison, C., Hashimoto, M., Utsumi, H. et al. Homologous recombination and non-homologous end-joining pathways of DNA double-strand break repair have overlapping roles in the maintenance of chromosomal integrity in vertebrate cells. EMBO J. 17, 5497–5508 (1998).
Essers, J., van Steeg, H., de Wit, J., Swagemakers, S. M., Vermeij, M., Hoeijmakers, J. H. et al. Homologous and non-homologous recombination differentially affect DNA damage repair in mice. EMBO J. 19, 1703–1710 (2000).
Fishel, M. L., Vasko, M. R. & Kelley, M. R. DNA repair in neurons: so if they don’t divide what's to repair? Mutat. Res. 614, 24–36 (2007).
Acknowledgements
We thank Ruoxin Zhang for providing help in revision and modification of this paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, T., He, J., Huang, Y. et al. The generation of mitochondrial DNA large-scale deletions in human cells. J Hum Genet 56, 689–694 (2011). https://doi.org/10.1038/jhg.2011.97
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2011.97
Keywords
This article is cited by
-
Multi-level profiling unravels mitochondrial dysfunction in myotonic dystrophy type 2
Acta Neuropathologica (2024)
-
Mitochondria DNA copy number, mitochondria DNA total somatic deletions, Complex I activity, synapse number, and synaptic mitochondria number are altered in schizophrenia and bipolar disorder
Translational Psychiatry (2022)
-
Repetitive DNA profile of the amphibian mitogenome
BMC Bioinformatics (2020)