Abstract
The aim of this study was to evaluate the relationship between smoking, alcohol drinking and genetic polymorphism of the growth hormone 1 gene (GH1) T1663A with reference to colorectal cancer. We conducted a case–control study with 315 cases of colorectal cancer and 438 population-based controls in the Jiangsu Province, China. GH1 T1663A genotypes were identified using PCR–RFLP (restriction fragment length polymorphism) methods. Information on smoking and drinking was collected using a questionnaire. Odds ratios (ORs) were estimated with an unconditional logistic model. The distribution of T/T and A/A genotypes was significantly different between controls and cases (χ2MH=3.877, P=0.049). Compared with the GH1 T/T genotype, the A/A genotype was at a decreased risk of developing colorectal cancer (sex-, age-, body mass index-, smoking- and alcohol drinking-adjusted OR=0.56, 95% confidence interval: 0.34–0.90). Smoking was not associated with the risk of colorectal cancer, whereas alcohol drinking was associated with an increased risk of colorectal cancer. Among nonsmokers or nondrinkers, individuals who had the GH1 A/A genotype were at a decreased risk of developing colorectal cancer compared with individuals who had the GH1 T allele. These results show that the GH1 T1663A A/A genotype can decrease the risk for colorectal cancer.
Similar content being viewed by others
Introduction
The growth hormone (GH) gene is associated with altered GH production. By binding to its receptor, GH1 stimulates the production of insulin-like growth factor-I (IGF-I) and its binding protein IGFBP-3, resulting in the regulation of cell proliferation, differentiation and apoptosis. IGF-1 is an important mitogen required for progression through the cell cycle. The GH/IGF-I axis has a clearly established role in somatic growth regulation, and may also contribute to neoplastic growth for several common cancers.1, 2, 3 Some epidemiological studies have investigated the role of IGFs and IGFBPs in the etiology of cancers of the breast, colon, rectum, prostate and lung, as well as of childhood leukemia, and have provided reasonably consistent support for increased risk of solid tumors in association with relatively high levels of IGF-I, decreased risk of solid tumors and childhood leukemia in association with relatively high levels of IGFBP-3, as well as increased risk of breast cancer in association with a high ratio of IGF-I to IGFBP-3.1 IGF-I mediates many of the physiological effects of GH, and plasma levels of IGF-I are associated with risk for colorectal cancer in healthy individuals.4, 5, 6 Patients with acromegaly, a disease characterized by abnormally high levels of GH secretion, are at an elevated risk for colorectal cancer.7, 8, 9 GH is the main determinant of circulating levels of IGF-I and its main binding protein, IGFBP-3.1 The GH1 gene is polymorphic and a substitution polymorphism (T1663A) is considered to be associated with GH production.10 To investigate possible relationships between GH1 T1663A polymorphisms (rs2 665 802) and environmental factors (habitual smoking and alcohol drinking) for risk of colorectal cancers, we conducted a population-based case–control study in the Jiangsu Province of China.
Materials and methods
Study subjects
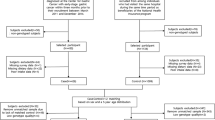
We recruited colorectal cancer cases using data obtained from the Cancer Registry in Huian and Jintan cities of the Jiangsu Province of China, and also recruited cases from patients who visited Jiangsu Provincial Cancer Hospital from these cities between August 2000 and September 2002. Cases were histopathologically diagnosed as having primary colorectal cancer. Physicians at the hospital or families of patients asked eligible cases to participate in our study, and doctors or nurses interviewed the subjects and collected their blood samples after obtaining informed consent. Population-based controls were selected from healthy residents in eight villages or towns of Huian and Jintan cities. Doctors of the public health center randomly selected one or two controls for each case, after matching for ethnicity, sex and age within 2 years of each case using the records of residents at the local governmental office, and then asked eligible residents for their participation, and conducted interviews and collected blood samples in the same manner. A total of 10 patients and 33 residents refused to participate in our study, but the final response rate was 97% for cases and 93% for controls. The ethical committee of the Jiangsu Provincial Institute of Cancer Research approved this study.
Environmental factors
Smoking and drinking habits were covered in our questionnaire. Each subject was asked whether he/she had ever smoked at least one cigarette per day for ⩾6 months. If he/she answered yes, he/she was further asked about the age at which he/she started to smoke cigarettes regularly, the average number of cigarettes smoked per day and the number of years he/she smoked. If the subject had quit smoking at least 1 year ago, the age at which he/she stopped smoking was recorded. Each subject was asked whether he/she had ever drunk alcoholic beverages at least once a month for ⩾1 year. If his/her answer was yes, he/she was asked to provide the age at which he/she started to drink regularly, as well as the frequency and usual amount of liquor, beer and grape wine consumed separately every time. If the subject had quit his/her drinking habit at least 1 year ago, the age at which he/she stopped drinking was recorded. Consumption of ethanol every month was calculated according to 40 g per 100 g of liquor, 3.5 g per 100 g of beer and 12 g per 100 g of grape wine.
DNA extraction and GH1 genotyping
Whole blood was collected into EDTA-coated tubes and centrifuged for 15 min, and the buffy coat layer was isolated. Genomic DNA was extracted from 200 μl of buffy coat using a Qiagen QIAamp DNA Blood Mini Kit (QIAGEN Companies, Germany). The PCR assay used to detect the T-to-A variant at position 1663 (T1663A) in intron 4 of the GH1 gene was a two-step method because of the close homologies that exist between GH1 and other related genes in the GH cluster.10 The primers for the first amplification were F4 (5′-GGCTGACCCAGGAGTCC-3′) and R1 (5′-AGAAGGACACCTAGTCAGACA-3′). Reactions were carried out in a total volume of 25 μl containing 12.5 pmol of each primer, 2.5 μl 4 × dNTPs, 1.5 μl 10 × buffer, 1 IU Tag polymerase and 1 μl genomic DNA. PCR conditions were 95 °C for 5 min, followed by 30 cycles of 95 °C for 1 min, 62 °C for 1 min and 72 °C for 30 s, with a final extension at 72 °C for 10 min. A volume of 3 μl of this product was further amplified with primers GH1MF2 (5′-GAGAAACACTGCTGCCCTCTTTTTAGACG-3′) and GH1R2 (5′-AAGAGAAGGAGAGGCCAAGC-3′) to produce a 180-bp product. The PCR product was subjected to AatII enzyme digestion in 37 °C for 3 h, and samples were then analyzed by electrophoresis in 3% agarose gels. The T allele was digested with AatII to fragments of 149 and 31 bp, whereas the A allele was not digested with AatII.
Statistical analysis
The body mass index (BMI) of subjects was calculated as weight (kg)/height (m2). The Mantel–Haenszel χ2-test was used to compare frequencies, and the t-test was used to compare means between cases and controls. The strength of associations between colorectal cancer and polymorphisms of GH1 was measured as odds ratios (ORs). ORs and their 95% confidence intervals were obtained using unconditional logistic regression analysis. We calculated adjusted ORs for age (continuous), sex, BMI (continuous), smoking and drinking habits. To investigate gene–environment interactions, we also calculated (stratified analysis) ORs according to combinations of GH1 genotypes and habits of smoking and drinking, with GH1 T allele carriers as reference. The procedure LOGISTIC from the statistical package SAS (SAS Institute Inc., USA) was used for the calculations. The probability of Hardy–Weinberg equilibrium was assessed by the χ2-test.
Results
A total of 190 male and 125 female cases with colorectal cancer, and 222 male and 216 female controls (Table 1), were included in this study. The proportional distribution of females in controls was significantly higher than that in colorectal cases. The mean age and BMI did not significantly differ between cases and controls. The proportional distributions of smokers and drinkers were significantly higher in colorectal cancer cases than in controls.
The distributions of GH1 T/T, T/A and A/A genotypes were 38.1, 45.9 and 16.0% in controls, and 42.2, 46.7 and 11.1% in colorectal cases, respectively (Table 1). The proportional distribution of T/T and A/A genotypes was significantly different between controls and colorectal cancer cases (χ2MH=3.877, P=0.049), but that of three groups was not significantly different (χ2MH=3.907, df=2, P=0.142). The frequencies of the A variant of the GH1 allele were 39% for controls and 34% for colorectal cancer cases, and were in Hardy–Weinberg equilibrium (χ2=0.532 and χ2=0.350, P-value >0.05). It shows that subjects from the population are representative. After adjusting for sex, age, BMI and habitual smoking and drinking, decreased OR for colorectal cancer (0.56, 95% confidence interval=0.34–0.90) was observed in individuals with the GH1 A/A genotype, when compared with the GH1 T/T genotype. Similar ORs were observed in stratified analyses for males and females and for colon and rectal cancer, although they were not statistically significant (Table 2).
Table 3 shows the relationship of smoking and alcohol drinking to colorectal cancer. There was no statistically significant association between smoking habit and colorectal cancer risk, whereas alcohol drinking showed significantly increased ORs for colorectal cancer.
Table 4 shows data for interactions between GH1 and habitual smoking and drinking for risk of colorectal cancer. The interactions between the GH1 polymorphism and status of smoking and alcohol drinking were not statistically significant (P for smoking=0.4277 and P for drinking=0.1226). Compared with nonsmokers or nondrinkers with the GH1 T allele, the GH1 A/A genotype showed decreased ORs both in nonsmokers (age-, sex-, BMI- and alcohol drinking-adjusted OR=0.47, 95% confidence interval=0.25–0.88) and nondrinkers (age-, sex-, BMI- and smoking adjusted-OR=0.56, 95% confidence interval=0.32–0.98).
Discussion
In this study, we detected that a polymorphism in the human GH1 gene might be associated with risk of colorectal cancer in Chinese, that is, the GH1 1663A/A genotype decreases the risk of colorectal cancer.
GH secretion is partially determined by polymorphisms in the GH1 gene; Hasegawa et al.11 observed that frequency of the A allele at A1663T in GH-insufficient children, normal short children and healthy normal-height adults was 57.0, 35.9 and 42.2%, respectively. The frequency of the polymorphism in the GH insufficiency group was significantly different from that in other groups. Six of eight patients with the severe type of GH insufficiency had the A/A genotype at A1663T, whereas two had the A/T genotype at A1663T (none had the T/T genotype). These results showed that the GH1 1663 A allele is associated with lower GH secretion. Le Marchand et al.10 has also found that the GH1 1663 A/A genotype is associated with a lower ratio of plasma IGF-I/IGFBP-3 and a higher level of IGFBP-1, which are consistent with a lower GH secretion. The carcinogenic role of IGFs in cancer is supported by epidemiological studies, which have found that high levels of circulating IGF-I and low levels of IGFBP-3 are associated with increased risk of several common cancers, including colorectal cancer, breast cancer, prostate cancer, ovarian cancer, lung cancer and childhood leukemia.1, 10 Le Marchand et al.10 have observed that the human GH1 gene T1663A polymorphism is associated with decreased risk of colorectal cancer in Caucasians and native Hawaiians. Khoury-Shakour et al.12 found that the A allele of the GH1 polymorphism is associated with reduced risk of colorectal cancer among physically inactive individuals, indicating an interaction between physical activity and the GH/IGF-I system. Our findings in this study are consistent with these previous results.
In stratified analyses, we found that both males and females with the GH1 1663A/A genotype have a decreased OR for colorectal, colon and rectal cancers, although it was not statistically significant.
In the previous study, we found that alcohol drinking is associated with increased risk of colorectal cancer, whereas smoking is not associated with risk of colorectal cancer.13, 14 In this study, we found that the protective effect of the GH1 1663A/A genotype for colorectal cancer was more notable in nonsmokers of cigarettes and nondrinkers of alcohol. The relationship between GH1 and habitual smoking and drinking is unclear, but GH1 increases production of both IGF-1 and IGFBP-3, accounting in part for the relatively high correlation between plasma IGF-1 and IGFBP-3. Our result showed that habitual smoking and drinking may affect the role of GH1, or may affect the serum levels of IGF-1 and IGFBP-3. There are already some studies on the association between habitual smoking and alcohol drinking and the serum levels of IGF-I and IGFBP-3,15, 16, 17, 18, 19, 20, 21 although those results are inconsistent. However, several studies have suggested that habits of smoking and alcohol drinking are associated with serum levels of IGF-1 and IGFBP-3. Kaklamani et al.15 observed that serum levels of IGF-1 were positively associated with pack-year history of smoking, and serum levels of IGF-BP3 were independently and negatively associated with the number of cigarettes per day or pack-year history of smoking. Clinical studies also showed that high-nicotine cigarettes increase serum GH levels.20, 21 Gapstur et al. found that greater alcohol intake and, to a lesser extent, greater number of cigarettes smoked per day, were associated with lower serum IGF-I concentrations in both Black and White men. Smoking was also inversely associated with IGFBP-3, but only in White men.16 Yu and Rohan1 noted that the associations of alcohol and cigarette smoking with IGF-I and IGFBP-3 could be mutually confounding. In this study, we found no significant interaction between habitual smoking or drinking and the GH1 T1663A polymorphism with regard to risk for colorectal cancer.
Finally, some limitations in this study require further discussion. The sample size in this study was not sufficient for stratified subgroup analyses, with consequent reduction in the magnitude of statistical power and increase in the potential for random error. Another possible problem is selection bias for controls, these being recruited by local health staff, although from the general population with a high response rate. The proportional distribution of females in controls was higher than that in colorectal cases, which may have caused a lower prevalence of smokers and alcohol drinkers in the present controls, although we adjusted for sex and age in all statistical analyses.
In summary, this study revealed a positive association between the GH1 T1663A polymorphism and decreased risk of colorectal cancer, with a significant interaction between the GH1 T1663A polymorphism and habitual smoking and alcohol drinking with regard to development of colorectal cancer. The data support the fact that colorectal cancer susceptibility with GH1 polymorphisms may be altered by background environmental factors.
References
Yu, H. & Rohan, T. Role of the insulin-like growth factor family in cancer development and progression. J. Natl Cancer Inst. 92, 1472–1489 (2000).
Yu, H., Spitz, M. R., Mistry, J., Gu, J., Hong, W. K. & Wu, X. F. Plasma levels of insulin-like growth factor-I and lung cancer risk: a case–control study. J. Natl Cancer Inst. 91, 151–156 (1999).
Holly, J. M., Gunnell, D. J. & Smith, G. Growth hormone, IGF-1 and cancer. Less intervention to avoid cancer? More intervention to prevent cancer? J. Endocrinol. 162, 321–330 (1999).
Ma, J., Pollak, M. N., Giovannucci, E., Chan, J. M., Tao, Y., Hennekens, C. H. et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J. Natl Cancer Inst. 91, 620–625 (1999).
Giovannucci, E., Pollak, M. N., Platz, E. A., Willett, W. C., Stampfer, M. J., Majeed, N. et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol. Biomarkers Prev. 9, 345–349 (2000).
Kaaks, R., Toniolo, P., Akhmedkhanov, A., Lukanova, A., Biessy, C., Dechaud, H. et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J. Natl Cancer Inst. 92, 1592–1600 (2000).
Jenkins, P. J., Fairclough, P. D., Richards, T., Lowe, D. G., Monson, J., Grossman, A. et al. Acromegaly, colonic polyps and carcinoma. Clin. Endocrinol. (Oxf) 47, 17–22 (1997).
Orme, S. M., McNally, R. J., Cartwright, R. A. & Belchetz, P. E. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J. Clin. Endocrinol. Metab. 83, 2730–2734 (1998).
Jenkins, P. J., Frajese, V., Jones, A. M., Camacho-Hubner, C., Lowe, D. G., Fairclough, P. D. et al. Insulin-like growth factor I and the development of colorectal neoplasia in acromegaly. J. Clin. Endocrinol. Metab. 85, 3218–3221 (2000).
Le Marchand, L., Donlon, T., Seifried, A., Kaaks, R., Rinaldi, S. & Wilkens, L. R. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J. Natl Cancer Inst. 94, 454–460 (2002).
Hasegawa, Y., Fujii, K., Yamada, M., Igarashi, Y., Tachibana, K., Tanaka, T. et al. Identification of novel human GH-1 gene polymorphisms that are associated with growth hormone secretion and height. J. Clin. Endocrinol. Metab. 85, 1290–1295 (2000).
Khoury-Shakour, S., Gruber, S. B., Lejbkowicz, F., Rennert, H. S., Raskin, L., Pinchev, M. et al. Recreational physical activity modifies the association between a common GH1 polymorphism and colorectal cancer risk. Cancer Epidemiol. Biomarkers Prev. 17, 3314–3318 (2008).
Gao, C. M., Takezaki, T., Wu, J. Z., Chen, M. B., Liu, Y. T., Ding, J. H. et al. CYP2E1 Rsa I polymorphism impacts on risk of colorectal cancer associated with smoking and alcohol drinking. World J. Gastroenterol. 13, 5725–5730 (2007).
Gao, C. M., Takezaki, T., Wu, J. Z., Zhang, X. M., Cao, H. X., Ding, J. H. et al. Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J. Gastroenterol. 14, 5078–5083 (2008).
Kaklamani, V. G., Linos, A., Kaklamani, E., Markaki, I. & Mantzoros, C. Age, sex, and smoking are predictors of circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3. J. Clin. Oncol. 17, 813–817 (1999).
Gapstur, S., Kopp, P., Chiu, B., Gann, P., Colangelo, L. & Liu, K. Longitudinal associations of age, anthropometric and lifestyle factors with serum total insulin-like growth factor-I and IGF binding protein-3 levels in black and white men: the CARDIA Male Hormone Study. Cancer Epidemiol. Biomarkers Prev. 13, 2208–2216 (2004).
Goodman-Gruen, D. & Barrett-Connor, E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am. J. Epidemiol. 145, 970–976 (1997).
Signorello, L. B., Kuper, H., Lagiou, P., Wuu, J., Mucci, L. A., Trichopoulos, D. et al. Lifestyle factors and insulin-like growth factor 1 levels among elderly men. Eur. J. Cancer Prev. 9, 173–178 (2000).
Lukanova, A., Toniolo, P., Akhmedkhanov, A., Hunt, K., Rinaldi, S., Zeleniuch-Jacquotte, A. et al. A cross-sectional study of IGF-I determinants in women. Eur. J. Cancer Prev. 10, 443–452 (2001).
Seyler, L. E. Jr, Pomerleau, O. F., Fertig, J. B., Hunt, D. & Parker, K. Pituitary hormone response to cigarette smoking. Pharmacol. Biochem. Behav. 24, 159–162 (1986).
Wilkins, J. N., Carlson, H. E., Van Vunakis, H., Hill, M. A., Gritz, E. & Jarvik, M. E. Nicotine from cigarette smoking increases circulating levels of cortisol, growth hormone, and prolactin in male chronic smokers. Psychopharmacology (Berl) 78, 305–308 (1982).
Acknowledgements
This study was supported in part by a Grant-in Aid for International Scientific Research; Special Cancer Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan, No. 11 137 311; and Major International (Regional) Joint Research Projects from the National Natural Science Foundation of China (NSFC), No. 30 320 140 461. We thank the staff of the Huaian City Municipal Hospital and Public Health Center of Huaian and Jintan Cities for their assistance in data collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, CM., Gong, JP., Wu, JZ. et al. Relationship between growth hormone 1 genetic polymorphism and susceptibility to colorectal cancer. J Hum Genet 55, 163–166 (2010). https://doi.org/10.1038/jhg.2010.3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2010.3