Abstract
Nevoid basal cell carcinoma syndrome (NBCCS) is a rare autosomal dominant disorder characterized by developmental abnormalities and a predisposition to cancers. Two unrelated patients, 21- and 16-year-old males, with cleft lip and palate and multiple jaw cysts, were diagnosed according to clinical criteria. To confirm a diagnosis of NBCCS, we undertook a molecular genetic analysis of the PTCH gene. Their PTCH genes were analyzed by direct sequencing of the PCR product from their DNA, and previously unreported mutations were identified. A heterozygous duplication at the nucleotide position between 3325 and 3328 of the PTCH gene (c.3325_3328dupGGCG) was detected in the 21-year-old patient. It caused a frameshift mutation, resulting in a premature termination of the PTCH protein. A point mutation (G to C) in intron 7 of the PTCH gene (c.1067+1G>C) was detected in the 16-year-old patient. This caused an aberrant splicing of PTCH. It is interesting to note that the non-canonical cryptic splice-donor site was activated, which did not conform to the GT–AG rule.
Similar content being viewed by others
Introduction
The nevoid basal-cell carcinoma syndrome (NBCCS) or the Gorlin syndrome is an autosomal dominant disorder first described by Gorlin and Goltz in 1960,1 and characterized by developmental abnormalities and tumorigenesis such as basal cell carcinoma and medulloblastoma. Pathognomonic signs including nevoid basal-cell carcinoma (BCC), multiple jaw cysts, palmar and plantar pits and calcification of the falx cerebri lead to the diagnosis of NBCCS. Its prevalence is estimated to be 1/56 000 in the United Kingdom to 1/164 000 in Australia.2, 3 However, it remains unreported from other countries including Japan. Complications have been described for NBCCS, including congenital skeletal anomalies such as bifid ribs, medulloblastoma, cleft lip and palate (CLP).2, 3 The human homolog of Drosophila patched (PTCH), mapped to the NBCCS locus on chromosome 9q22.3, has been found to be mutated in patients with NBCCS in 1996.4, 5 It is reported that 75% of Japanese NBCCS patients had a PTCH mutation.6 Although some authors reported NBCCS associated with CLP,7, 8, 9, 10 mutational analysis was poorly performed in NBCCS associated with CLP.11 CLP is a major congenital anomaly, with a birth prevalence from 0.3/1000 in African American to 3.6/1000 in Amerindian, depending on genetic, geographic, ethnic and socioeconomic factors.12 The incidence of CLP in Japanese newborns is approximately 2.1 in 1000.12 It is reported that the incidence of CLP in NBCCS is 4–9.1%.2, 3, 9, 13 There is an excess of males with CLP, the proportion ranging from 60 to 80 percent.14 The male–female ratio of clefting in this syndrome is statistically not different from the ratio in the normal population.10 To confirm a diagnosis of NBCCS, we undertook a molecular genetic analysis of the PTCH gene in two unrelated NBCCS patients with CLP.
Materials and methods
Patients
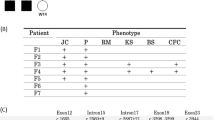
Two unrelated patients with CLP showed multiple jaw cysts in a panoramic X-ray during a series of cleft lip and palate treatments, and were clinically diagnosed as having NBCCS by the clinical criteria of Kimonis et al.15 (Table 1). Patient 1, a 21-year-old male, showed palmar and plantar pits, calcification of the falx cerebri, bridging of sella, bifid ribs, scoliosis and Sprengel shoulder (Figure 1). Patient 2, a 16-year-old male, showed calcification of the falx cerebri and bridging of sella. Neither patient showed BCC and a family history of NBCCS. Both the patients underwent a multiple jaw cysts removal operation. Pathological finding indicated a keratocystic odontogenic tumor (KCOT) (Figure 2).
Patient 2, a 16-year-old male. (a) Panoramic X-ray shows five multiple jaw cysts. (b) Operative view. (c) Removed cyst from the asterisk of A. Scale bar: 10 mm. (d) HE staining shows lining of parakeratinized squamous epithelium. It indicates a keratocystic odontogenic tumor (KCOT). Scale bar: 50 μm.
Mutational analysis
All studies described below were approved by the ethics committee at Kitasato University. After written informed consent was obtained, blood samples were taken from each patient. Genomic DNA was extracted using a QIAamp DNA blood midi kit. For the production of the Epstein–Barr virus (EBV)-immortalized lymphoblastoid cell line, mononuclear cells were washed and suspended in an RPMI-1640 medium with 20% fetal calf serum (1.2 × 106 ml). The cells were cultured in multiple aliquots in 24-well tissue culture plates (1 × 106 per well) with a 20% filtrate of a B95-8 culture supernatant. The cultures were refed and expanded for 3–6 weeks and harvested for the preparation of total RNA. Genomic DNA samples were amplified with primers for all exons as described previously.6 Amplified products were gel-purified using a QIAEX II gel extraction kit (Qiagen, Valencia, CA, USA) and cycle sequenced with a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA) in both directions. The sequence was analyzed on a 3130 Genetic Analyzer (Applied Biosystems). For the analysis of aberrant splicing, total RNA was extracted from an EBV-immortalized lymphoblastoid cell line using the QIAamp RNA Blood Mini Kit (QIAGEN) and subjected to reverse-transcription (RT)-PCR using a QIAGEN LongRange 2Step RT-PCR Kit (QIAGEN) with oligo dT. The forward and reverse primers for exon 6 and 9 were 5′-CTTCGACCCTTTGGAATTCCTGG-3′ and 5′-TCACAGGGTCGTGGTGGTGAAGGAAA-3′, respectively. The amplified product was also sequenced as described above.
Results
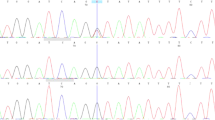
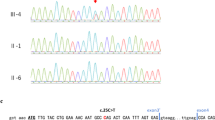
A heterozygous duplication at the nucleotide position between 3325 and 3328 of PTCH exon 20 (c.3325_3328dupGGCG) was detected in patient 1 (Figure 3a). It caused a frameshift mutation and a prematurely terminated translation of the PTCH gene. In patient 2, a point mutation (G to C) in intron 7 of the PTCH gene (c.1067+1G>C) was detected (Figure 3b). To analyze the splicing events in this patient, total RNA was extracted from the EBV-immortalized lymphoblastoid cell line, and an RT-PCR analysis was performed using a pair of primers located in exon 6 and exon 9 (Figure 3b). As shown in Figure 3c, an additional PCR product of larger molecular weight was detected in patient 2 (transcript 2). Sequencing this product revealed that this mutation caused the activation of a cryptic splice site in intron 7, resulting in a 77-bp insertion of the intronic sequence (Figures 3b and c), which also prematurely terminated the PTCH protein because of the in-frame stop codon located just after the mutation. This activated splice site did not conform to the GT–AG rule.16
Mutations found in our study. (a) A heterozygous duplication at the nucleotide position between 3325 and 3328 of the PTCH gene (c.3325_3328dupGGCG) was detected in patient 1. (b) A point mutation (G to C) in intron 7 of the PTCH gene (c.1067+1G>C) was detected in patient 2. Red arrows indicate the position of primers used for RT-PCR (See also c). (c) Electropherogram of the RT-PCR products. In addition to the normal transcript (transcript 1), a longer product (transcript 2) was detected in patient 2. Sequencing this product revealed the aberrant splicing that lead to the addition of the intron 7 sequence of 77 bp (See also b). A full colour version of this figure is available at the Journal of Human Genetics journal online.
Discussion
Multiple jaw cysts were the first signs of NBCCS in these two cases and the pathological finding indicated KCOT. It is reported that the incidence of KCOT in NBCCS is 75–90%.2, 3, 13 KCOT, previously known as odontogenic keratocyst, is a benign cystic lesion, but it often shows locally destructive behavior and a high recurrence rate.17 Therefore, in the new WHO classification revised in 2005, KCOT is defined as a benign neoplasm of odontogenic origin with the characteristic lining of a parakeratinized squamous epithelium.18 Therefore, a careful clinical observation is needed for patients with KCOT.
CLP is a common birth defect that results from a mixture of genetic and environmental factors, and two genes, MSX1 and IRF6, now seem to have a measurable role in the causation of CLP.19, 20 It is reported that the incidence of CLP in NBCCS is 4–9.1%.2, 3, 9, 13 The human homolog of Drosophila patched (PTCH) has been found to be mutated in patients with NBCCS.4, 5 The PTCH protein is a receptor for Sonic hedgehog (SHH). SHH is essential for the morphogenesis of frontonasal and maxillary processes and the loss of SHH signaling in the embryonic face inhibits the growth of the primordial and results in defects of CLP.21 However, mutational analysis was poorly performed in NBCCS associated with CLP. Therefore, it is not clear whether the PTCH gene mutation specifically causes CLP.11
Thus, we undertook PTCH mutational analysis and identified two previously unreported mutations in PTCH. Mutational analysis is also important to confirm the clinical diagnosis of NBCCS and to assess and to reduce the risk for BCC. BCC is one of the major criteria of NBCCS, and is reported to occur in 75% of patients over 20 years of age and in more than 90% over 40 years of age in the United Kingdom.2 The prevalence of BCC is 15% lower in Korean NBCCS patients than in those from the United Kingdom, probably due to the difference in the degree of skin pigmentation.13 As face and back are the most severely affected sites of BCCs,3 ultraviolet radiation is a risk factor for developing BCCs.
Interestingly, in patient 2, a cryptic splice site that does not conform to the GT–AG rule was activated at least in the EBV-immortalized lymphoblastoid cell line, because the second base of intron 7, which was created by the aberrant splicing, was cytosine, not thymine. This cryptic splice site cannot be predicted by current algorithm such as the one proposed by Sahashi et al.22 Although the reason why this occurred is unknown, this case tells us that the exact nature of aberrant splicing resulting from splice site mutations is unlikely to be predicted. Therefore, an RNA analysis such as RT-PCR is indispensable in the detection of abnormal mRNA if a mutation that potentially affects RNA splicing is identified.
References
Gorlin, R. J. & Goltz, R. W. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N. Engl. J. Med. 262, 908–912 (1960).
Evans, D. G. R., Ladusan, E. J., Rimmer, S., Burnell, L. D., Thakker, N. & Farndon, P. A. Complications of the nevoid basal cell carcinoma syndrome: results of the population based study. J. Med. Genet. 30, 460–464 (1993).
Shanley, S., Ratcliffe, J., Hockey, A., Haan, E., Oley, C., Ravine, D. et al. Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am. J. Med. Genet. 50, 282–290 (1994).
Hahn, H., Wicking, C., Zaphiropoulous, P. G., Gailani, M. R., Shanley, S., Chidambaram, A. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Johnson, R. L., Rothman, A. L., Xie, J., Goodrich, L. V., Bare, J. W., Bonifas, J. M. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671 (1996).
Fujii, K., Kohno, Y., Sugita, K., Nakamura, M., Moroi, Y., Urabe, K. et al. Mutations in the human homologue of Drosophila patched in Japanese nevoid basal cell carcinoma syndrome patients. Hum. Mutat. 21, 451–452 (2003).
Taicher, S. & Shteyer, A. The basal cell nevus syndrome associated with cleft lip and cleft palate: report of case. J. Oral. Surg. 36, 799–802 (1978).
Soekarman, D., Fryns, J. P., Casaer, P. & Van Den Berghe, H. Increased head circumference and facial cleft as presenting signs of the nevoid basal-cell carcinoma syndrome. Genet. Couns. 2, 157–162 (1991).
Lambrecht, J. T. & Kreusch, T. Examine your orofacial cleft patients for Gorlin–Goltz syndrome. Cleft. Palate. Craniofac. J. 34, 342–350 (1997).
van Dijk, E. & Neering, H. The association of cleft lip and palate with basal cell nevus syndrome. Oral. Surg. Oral. Med. Oral. Pathol. 50, 214–216 (1980).
Mansilla, M. A., Cooper, M. E., Goldstein, T., Castilla, E. E., Lopez Camelo, J. S., Marazita, M. L. et al. Contributions of PTCH gene variants to isolated cleft lip and palate. Cleft. Palate. Craniofac. J. 43, 21–29 (2006).
Noordhoff, M. S. & Chen, P. K. Unilateral Cheiloplasty-Plastic Surgery 2nd edn (Saunders, Philadelphia, 2006).
Ahn, S. G., Lim, Y. S., Kim, D. K., Kim, S. G., Lee, S. H. & Yoon, J. H. Nevoid basal cell carcinoma syndrome: a retrospective analysis of 33 affected Korean individuals. Int. J. Oral. Maxillofac. Surg. 33, 458–462 (2004).
McCarthy, J. G., Cutting, C. B. & Hogan, V. M. Introduction to Facial Clefts—Plastic Surgery (Saunders, Philadelphia, 1990).
Kimonis, V. E., Goldstein, A. M., Pastakia, B., Yang, M. L., Kase, R., DiGiovanna, J. J. et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am. J. Med. Genet. 69, 299–308 (1997).
Breathnach, R. & Chambon, P. Organization and expression of eukaryotic split genes coding for proteins. Annu. Rev. Biochem. 50, 349–383 (1981).
Morgan, T. A., Burton, C. C. & Qian, F. A retrospective review of treatment of the odontogenic keratocyst. J. Oral. Maxillofac. Surg. 63, 635–639 (2005).
Philipsen, H. P. Keratocystic odontogenic tumour. in World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours (eds Barnes, L., Eveson, J.W., Reichart, P.A. & Sidransky, D.) 306–307 (IARC Press: Lyon, 2005).
van den Boogaard, M. J., Dorland, M., Beemer, F. A. & van Amstel, H. K. MSX1 mutation is associated with orofacial clefting and tooth agenesis in humans. Nat. Genet. 24, 342–343 (2000).
Zucchero, T. M., Cooper, M. E., Maher, B. S., Daack-Hirsch, S., Nepomuceno, B., Ribeiro, L. et al. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N. Engl. J. Med. 351, 769–780 (2004).
Hu, D. & Helms, J. A. The role of sonic hedgehog in normal and abnomal craniofacial morphogenesis. Development 126, 281–292 (1999).
Sahashi, K., Masuda, A., Matsuura, T., Shinmi, J., Zhang, Z. & Takeshima, Y. In vitro and in silico analysis reveals an efficient algorithm to predict the splicing consequences of mutations at the 5′ splice sites. Nucleic. Acids. Res. 35, 5995–6003 (2007).
Acknowledgements
We are indebted to the patients who participated in this study. We also thank Hiromi Hatsuse for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasaki, R., Saito, K., Watanabe, Y. et al. Nevoid basal cell carcinoma syndrome with cleft lip and palate associated with the novel PTCH gene mutations. J Hum Genet 54, 398–402 (2009). https://doi.org/10.1038/jhg.2009.51
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2009.51