Abstract
Chronic granulomatous disease (CGD) is an immunodeficiency caused by defects in the nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase complex and is usually diagnosed in early childhood. CGD patients suffer from severe, recurrent infections with bacteria, fungi and yeasts. We report a 25-year-old female with protracted fever because of a Staphylococcus aureus liver abscess, which did not resolve until breakthrough into the stomach. Despite her age, CGD was considered on diagnosis on the basis of the clinical symptoms. Analysis of the NADPH–oxidase activity confirmed CGD as the underlying condition. Western blotting revealed the absence of p47phox and subsequent sequencing of the p47phox-encoding gene, neutrophil cytosolic factor (NCF1), identified a deletion of 837C in the maternal NCF1 allele. The paternal allele contained a stopcodon because of a conversion between NCF1 and one of its ΨNCF1 pseudogenes. The patient had one novel mutation, c.837delC, and one conversion in NCF1, resulting in the complete absence of the p47phox component of the NADPH–oxidase complex. This p47phox-deficient CGD patient had the highest age at diagnosis reported thus far.
Similar content being viewed by others
Introduction
Chronic granulomatous disease (CGD) is a rare disease caused by defects in nicotinamide adenine dinucleotide phosphate (NADPH)–oxidase, the enzyme complex that generates superoxide. Phagocytic leukocytes produce superoxide in the phagosome, where it is converted into microbicidal reactive oxygen metabolites, such as H2O2, which are essential for killing various bacteria, fungi and yeasts. The vast majority of CGD patients are diagnosed in their first 5 years of life because of persistent or recurrent, severe, life-threatening infections with Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia species and Aspergillus species.1 In CGD patients, defects have been reported in five of the NADPH–oxidase subunits: gp91phox p47phox p22phox p67phox and Rac2.2, 3 Most male CGD patients have a defect in the X-linked CYBB gene for gp91phox , whereas the majority of female patients are found to have a defect in the autosomal neutrophil cytosolic factor 1 (NCF1) gene for p47phox. The most common mutation in NCF1, the gene encoding p47phox, is due to a conversion of NCF1 with one of two NCF1 pseudogenes (ΨNCF1), resulting in a deletion of two nucleotides and a premature termination of protein synthesis. Here, we report an adult patient who was compound heterozygous for one novel mutation and one classical conversion in NCF1.
Materials and methods
Patient
A 25-year-old female was hospitalized because of pain in the right upper quadrant of the abdomen and intermittent fever of 6 weeks duration. Besides mild recurrent folliculitis in both axillae and impetigo on atopic eczema since adolescence, her earlier medical history was unremarkable. On hospitalization, she complained of progressive, stabbing pain in the right upper quadrant of the abdomen. She had chills and an intermittent fever in the past 6 weeks. Owing to malaise and decreased appetite, she had lost weight. Moreover, the patient had noticed a tender nodule in the right axilla.
On physical exam, she did not appear ill and had a temperature of 37.3 °C with normal pulse and blood pressure. A nodule of about 2.5 cm diameter with central fluctuation, but lacking calor and redness, was palpable in the right axilla. Otherwise, besides mild tenderness on palpation of the right upper quadrant of the abdomen, the exam was unremarkable. The laboratory findings revealed an elevated erythrocyte sedimentation rate of 121 mm in the first hour, a C-reactive protein of 191 mg l−1 and a leukocytosis of 13.1 × 109/l. The blood glucose level was normal.
An echography of the liver showed a hypoechogenic lesion of 8–9 cm diameter in the left lobe, compatible with an abscess; this was confirmed by computed tomography scanning (Figure 1a). Blood cultures did not reveal growth of bacteria; a culture from the right axilla nodule grew S. aureus, methicillin sensitive. The liver abscess was drained percutaneously and thick pus was obtained; the culture yielded S. aureus. The abscess was rinsed with saline and treatment was initiated with flucloxacillin 12 g by continuous intravenous infusion. Owing to the difficulty of draining the thick pus, rifampicin 600 mg b.i.d. was added for 1 week. The diameter of the abscess did not change. Not until 1 month later did the diameter of the abscess reduce slightly and an injection of contrast into the drain reveal a fistula with breakthrough into the stomach (Figure 1b). Subsequently, her condition improved, and treatment was changed into flucloxacillin 1 g intravenously q.i.d., and after another month into flucloxacillin 500 mg q.i.d. orally. She made an uneventful recovery, and 1 year later, a computed tomography scan did not reveal any abnormalities in the abdomen.
Owing to recurrent hydradenitis—despite measures, such as mupirocin and use of betadine iodine—she was referred to our hospital for evaluation of the immune function. The clinical course led to the differential diagnosis of CGD.
NADPH oxidase assays
To assay the production of intracellular oxygen radicals, 2 × 106/ml polymorphonuclear neutrophils, isolated from peripheral blood using Ficoll gradient centrifugation in RPMI, 10% inactivated fetal calf serum (Gibco, Carlsbad, CA, USA) and 1 mg ml-1 4-NitroBlue Tetrazolium (Roche, Basel, Switzerland) were stimulated for 10 min at 37 °C and 4 r.p.m. with 10 ng ml-1 phorbol 12- myristate 13-acetate (Sigma). The cells were transferred by Cytospin to glass slides and stained with Giemsa (Merck, Whitehouse Station, NJ, USA). They were analyzed by light microscopy. The production of extracellular H2O2 was assayed in 5 × 105/ml polymorphonuclear neutrophils or peripheral blood mononuclear cells from peripheral blood in phosphate-buffered saline, 0.1% w/v glucose, 0.5 mM MgCl2, 0.9 mM CaCl2, 100 μM homovanillic acid (Fluka, St Louis, MO, USA) and in 1 U ml−1 horseradish peroxidase (Sigma, St Louis, MO, USA). The cells were stimulated with 10 ng ml−1 phorbol 12-myristate 13-acetate for 30 min at 37 °C and 4 r.p.m., or were preincubated for 10 min with 5 μg ml−1 cytochalasin E (Sigma) and stimulated with 1 μM n-formyl-methionyl-leucyl-phenylalanine (fMLP) for 30 min at 37 °C and 4 r.p.m. The reactions were stopped by adding a stopbuffer to a final concentration of 5.1 mM glycine, 5.1 mM NaCl, 6 mM NaOH and 2.8 mM EDTA. H2O2 concentration was determined in a fluorescence spectrophotometer (Hitachi F-4500, Hitachi, Tokyo, Japan).
Western blot of p47phox
A total of 107 peripheral blood mononuclear cells isolated from peripheral blood were lysed for 10 min at 4 °C in 5.7 mM diisopropylfluorophosphate (Fluka), phosphate-buffered saline. Protein was precipitated and resuspended in 10 mM EDTA, complete mini protease inhibitor mix (Roche) and incubated at 95 °C for 30 min. An equal volume of Laemmli loading dye (20% glycerol, 100 mM Tris pH 8.0, 4% SDS, 8% β-mercaptoethanol, 20% bromophenolblue) was added before loading and electrophoresis of the samples in a 12.5% SDS-polyacrylamide gel. The proteins were transferred by semi-dry electroblotting (Transblot, BioRad, Hercules, CA, USA) in a Towbin buffer (25 mM Tris, 192 mM glycine, pH 8.3) to a PVDF membrane (Perkin Elmer, Waltham, MA, USA). The membranes were blocked for 1 h with 2.5% ELK milk powder in a Tris-buffered saline, 0.01% Tween-20 (TBST). To detect p47phox, membranes were incubated for 1 h with 1/100 diluted mouse-anti-human p47phox antibody A-7 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in 1.25% ELK milk powder in TBST followed by three washes of 30 min with TBST. Incubation for 1 h with 1/1000 diluted secondary antibody goat-anti-mouse F(ab′)2-HRP (Santa Cruz) was followed by three washes of 30 min with TBST. To detect Actin, membranes were incubated for 1 h with 1/1000 diluted HRP-labeled goat-anti-human Actin antibody C-11 (Santa Cruz Biotechnology) followed by three washes of 30 min with TBST. Antibodies were detected with the ECL Western Blotting Detection System (Amersham, Chalfont St Giles, UK) according to the manufacturer's instructions.
Genetic analysis
RNA was isolated from whole blood with Trizol (Invitrogen, Carlsbad, CA, USA), and cDNA was generated with M-MLV Reverse Transcriptase (Invitrogen), both according to the manufacturer's instructions. NCF1 and ΨNCF1 transcripts were amplified with primers annealing to both transcripts and NCF1 transcripts were amplified with NCF1-specific primers.4 PCR products were sequenced with a Big Dye Terminator v 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA), and sequence reactions were analyzed on an ABI PRISM3700 DNA Analyzer (Applied Biosystems). DNA was isolated from whole blood essentially as described,5 exons were amplified (primers and assay conditions available upon request) and sequenced as described above.
To determine NCF1 and NCF1 pseudogene (ΨNCF1) ratios the gene-scan method was applied; a PCR was performed on DNA with primers that anneal in both the NCF1 and ΨNCF1 gene pseudogenes around the GT(GT) sequence in exon 2. This PCR results in 204-bp NCF1 and 202-bp ΨNCF1 PCR products that were analyzed on an ABIPRISM 3130XL Genetic Analyzer (Applied Biosystems). The gene-scan method, all conditions and primers have essentially been described in the study by Dekker et al.6
Results
To assay the function of the NADPH–oxidase complex, neutrophils were purified from blood and a nitroblue tetrazolium slide test was performed. In none of the cells from the patient production of formazan was observed in response to stimulation with phorbol myristate acetate. Both parents and a control showed a normal NBT test (Table 1). H2O2 production by peripheral blood mononuclear cells and neutrophils in response to fMLP or phorbol myristate acetate was analyzed and found to be normal in two controls but completely absent in the patient (Table 1).
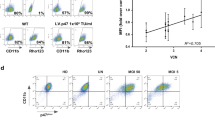
Next, protein isolated from neutrophils was analyzed on a western blot with the monoclonal antibody A-7 (Santa Cruz Biotechnology) directed against one of the NADPH–oxidase complex subunits; human p47phox In neutrophils from the parents and an unrelated healthy control p47phox protein was present while in neutrophils from the patient this was undetectable (Figure 2a).
(a) Western blot with the A-7 antibody directed against p47phox revealed that the protein was absent in the patient (P), and present in the mother (M), the father (F) and a healthy control (C). The C-11 antibody directed against actin shows that similar amounts of protein were present. (b) Sequence chromatograms of the region surrounding nucleotide 837 of the NCF1 gene in the father, the patient and the mother. Sequence reactions shown were performed with the reverse-primer. (c) Gene scan analysis of DNA to determine the ratio of NCF1 and ΨNCF1 genes in a healthy control, the mother, the father and the patient.
In order to identify mutations in NCF1, the gene encoding p47phox NCF1 and ΨNCF1 transcripts were amplified from RNA. In RNA from the patient and the parents both NCF1 and ΨNCF1 transcripts were detected. Sequencing of the NCF1 gene revealed a deletion of one nucleotide, c.837delC, in exon 9 in the patient (Figure 2b). This mutation leads to a frameshift in codon 280 and a premature stopcodon, designated as L280fsX374. In the NCF1 transcripts of the mother this mutation was present heterozygously (Figure 2b), and the presence of the mutation was confirmed in genomic DNA of the patient and her mother. The c.837delC mutation was not present in the NCF1 RNA (Figure 2b) or DNA of the father. The ratio of NCF1 and ΨNCF1 genes was subsequently determined in DNA of the patient, both parents and a healthy control with the gene-scan method.6 This quantitative analysis revealed that the mother and the control both had ratios of around 2 (Figure 2c), indicating they each had four pseudogenes and two NCF1 genes. The patient and the father both had ratios of around 5 (Figure 2c), indicating they each had five pseudogenes and only one NCF1 gene.
Discussion
We report a patient with a late onset of disease manifestations and unusual clinical presentation of CGD due to a novel mutation, c. 837delC, leading to a premature stopcodon in the NCF1 gene that encodes the p47phox subunit of NADPH oxidase.
Sixty-nine patients with mutations in NCF1 have been reported thus far (NCF1 mutation database: http://bioinf.uta.fi/NCF1base/),7 in these patients the most common mutation (present in 56 individuals) is caused by a conversion between NCF1 and an ΨNCF1 gene which is usually referred to as the 75_76delGT mutation. Only 15 other mutations affecting NCF1 have been reported (five affecting splicing and ten affecting the amino-acid sequence directly).8 Our patient was found to be compound heterozygous for the 75_76delGT mutation and a novel mutation, c.837delC, which to our knowledge has not been reported before. Both mutations result in frameshifts and premature termination of protein synthesis, accounting for the complete absence of p47phox protein and the complete defect observed in the functional assays.
Although liver abscesses are regularly observed in CGD patients, these are unusual as late first presentation of CGD, that is rarely occur in the third decade of life. According to an American registry of CGD patients (based on 368 patients) only 4% of CGD patients are diagnosed with CGD in the third decade of life.9 In the literature, only three p47phox-deficient patients have been reported who were diagnosed with CGD in their third decade of life. These were a 21-year old male with infections starting early in life that were not typical for CGD, thus postponing the correct diagnosis, a 22-year old female with infections starting early in life that were typical of CGD,8 and a 20-year old male with unspecified infections.10 Thus, the p47phox-deficient patient we describe here has the highest age at diagnosis reported thus far.
Most patients with a pyogenic liver abscess have an underlying condition, such as diabetes mellitus, chronic alcoholism or malignancy, or suffer from biliary or bowel disease, such as cholelithiasis or diverticulitis.11 In many patients, however, the cause of the abscess remains uncertain. Patients usually present acutely with a spiking fever and right hyponchondrial pain. Escherichia coli, Klebsiella pneumonia and S. milleri are the most frequent etiologic microbial agents.11 Rarely, a hepatogastric fistula develops. Our case underscores that in all infectious patients, including adults, who present with a protracted course and with an unusual etiological pathogen, that is, S. aureus, a diagnosis of underlying immunodeficiency, and CGD in particular, should be considered.
References
Rosenzweig, S. D. & Holland, S. M. Phagocyte immunodeficiencies and their infections. J. Allergy Clin. Immunol. 113, 620–626 (2004).
Heyworth, P. G., Curnutte, J. T., Rae, J., Noack, D., Roos, D., van Koppen, E. et al. Hematologically Important Mutations: X-Linked Chronic Granulomatous Disease (Second Update). Blood Cells Mol. Dis. 27, 16–26 (2001).
Cross, A. R., Noack, D., Rae, J., Curnutte, J. T. & Heyworth, P. G. Hematologically important mutations: the autosomal recessive forms of Chronic Granulomatous Disease (first update). Blood Cells Mol. Dis. 26, 561–565 (2000).
de Boer, M., Singh, V., Dekker, J., Di Rocco, M., Goldblatt, D. & Roos, D. Prenatal diagnosis in two families with autosomal, p47(phox)-deficient chronic granulomatous disease due to a novel point mutation in NCF1. Prenat. Diagn. 22, 235–240 (2002).
Sambrook, J. & Russell, D. W. Molecular cloning: a laboratory manual 3 edCold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, 2001.
Dekker, J., de Boer, M. & Roos, D. Gene-scan method for the recognition of carriers and patients with p47phox-deficient autosomal recessive chronic granulomatous disease. Exp. Hematol. 29, 1319–1325 (2001).
Piirilä, H., Väliaho, J. & Vihinen, M. Immunodeficiency mutation databases (IDbases). Hum. Mutat. 27, 1200–1208 (2006).
Roos, D., de Boer, M., Köker, M. Y., Dekker, J., Singh-Gupta, V., Ahlin, A. et al. Chronic granulomatous disease caused by mutations other than the common GT deletion in NCF1, the gene encoding the p47phox component of the phagocyte NADPH oxidase. Hum. Mutat. 27, 1218–1229 (2006).
Winkelstein, J. A., Marino, M. C., Johnston, R. B. Jr, Boyle, J., Curnutte, J., Gallin, J. I. et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79, 155–169 (2000).
El Kares, R., Barbouche, M., Elloumi-Zghal, H., Bejaoui, M., Chemli, J., Mellouli, F. et al. Genetic and mutational heterogeneity of autosomal recessive chronic granulomatous disease in Tunisia. J. Hum. Genet. 51, 887–895 (2006).
Seeto, R. K. & Rockey, D. C. Pyogenic liver abscess. Changes in etiology, management, and outcome. Medicine (Baltimore) 75, 99 (1996).
Acknowledgements
We thank the colleagues of the radiology department of the Medical Center Alkmaar for making the computed tomography-scan images available.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van de Vosse, E., van Wengen, A., van Geelen, J. et al. A novel mutation in NCF1 in an adult CGD patient with a liver abscess as first presentation. J Hum Genet 54, 313–316 (2009). https://doi.org/10.1038/jhg.2009.24
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jhg.2009.24