Abstract
Bone mineral density (BMD) is a major factor for determining bone strength and osteoporotic fracture risk, and is determined by environmental and multiple genetic factors. KIT, which encodes a transmembrane receptor with tyrosine kinase activity, plays an important role in the differentiation of osteoclasts. We examined the associations between KIT gene polymorphisms and BMD in postmenopausal Korean women. All exons, their boundaries, and the promoter region (approximately 1.5 kb) from 24 individuals were directly sequenced. Eighteen polymorphisms were identified, and three single-nucleotide polymorphisms (SNPs) were genotyped in all study participants (n = 946). BMD at the lumbar spine and femoral neck was measured using dual-energy X-ray absorptiometry. The mean age of the study subjects was 58.9 ± 7.5 years, and the mean number of years since menopause was 9.6 ± 7.9 years. None of the three SNPs (−1694G>T, +41894A>G, and +49512G>A) was significantly associated with BMD value. However, multivariate analysis showed that the ht3 (−1694T-+41894A-+49512G) was significantly associated with lower BMD at the femoral neck (P = 0.007 in the recessive model). These findings indicate that KIT-ht3 may be a useful genetic marker for osteoporosis and that KIT may have a role on bone metabolism in humans.
Similar content being viewed by others
Introduction
Osteoporosis is a systemic bone disease that is characterized by low bone mineral density (BMD) and increased risk of fractures. Among the multiple risk factors that affect BMD, and thus the pathogenesis of osteoporosis, genetic factors are most often involved and account for about 50–86% of variance in BMD according to twin and family studies (Arden and Spector 1997; Slemenda et al. 1991). Low BMD results from an imbalance between osteoclastic bone resorption and osteoblastic bone formation. Therefore, genes that are involved in osteoclast differentiation and function are potentially candidate genes for osteoporosis.
Osteoclast progenitors originate from hematopoietic stem cells (Boyle et al. 2003). Although the discovery of receptor activation of nuclear factor kappa B (NF-κB) pathways has shifted interest away from tyrosine kinase receptors (Lacey et al. 1998), the receptor tyrosine kinase for macrophage colony-stimulating factor (M-CSF), which is encoded by the FMS gene, is also important for osteoclast differentiation (Suda et al. 1997), and the closely related tyrosine kinase receptors, including those encoded by the KIT, MET, and FLT3 genes, which are present on pre-osteoclasts, can augment activation of these pathways (Gattei et al. 1996; Grano et al. 1996; Lean et al. 2001).
KIT, which is located on 4q11-q12, is the human homolog of the proto-oncogene c-kit, which encodes a transmembrane receptor with tyrosine kinase activity (Yarden et al. 1987), and its specific ligand has been identified as a growth factor called stem-cell factor (SCF) (Zsebo et al. 1990). It has been reported that KIT signaling might have a role in bone metabolism. The KIT gene has been found in human osteoclasts and pre-osteoclast cell lines, and mediates cell-to-cell interactions between osteoclasts and osteoblasts/stromal cells through membrane-bound SCF (Gattei et al. 1996), allowing more efficient and localized stimulation by specific cytokines and growth factors such as M-CSF and interleukin-1, which are key factors for osteoclastogenesis (Lowry et al. 1992). In addition, together with other factors, soluble SCF can expand the pool of osteoclast precursors (Demulder et al. 1992) and stimulate osteoclast formation and activity (van’t Hof et al. 1997). Furthermore, an altered response in tooth egression-induced bone remodeling was observed in c-kit mutant mice with partial loss of function (Silberstein et al. 1991). However, to our knowledge, there is no clinical evidence that the KIT gene has a role in regulation of bone metabolism. To investigate its role in the determination of bone mass in humans, we performed extensive screening of the KIT gene by direct sequencing to detect its polymorphisms, and we analyzed their associations with BMD in postmenopausal women.
Materials and methods
Subjects
The study population comprised 946 postmenopausal women of Korean ethnicity who visited the Asan Medical Center (AMC, Seoul, Korea). Menopause was defined as the absence of menstruation for at least 6 months and was confirmed by measurement of the serum levels of follicle-stimulating hormone (FSH). Women with premature menopause (before the age of 40 years) were excluded from the study. Women who had taken drugs that could affect bone metabolism for more than 6 months or within the previous 12 months were also excluded. Subjects were excluded if they had suffered from any disease that might affect bone metabolism. Women with previous stroke or dementia were also excluded because of concerns regarding their limited physical activity. Women were also excluded if they had osteophyte formation above the fourth grade of the Nathan classification (Nathan et al. 1994) and/or severe facet joint osteoarthritis in the lumbar spine that was diagnosed using conventional spine radiographs. The study was approved by the AMC ethics review committee, and written informed consent was obtained from all subjects.
BMD measurement
The BMD (g/cm2) of the anterior–posterior lumbar spine (L2–L4) and femoral neck was measured using dual-energy X-ray absorptiometry. A Lunar Expert XL scanner with software version 1.90 (Madison, WI) was used in 637 women, and a Hologic QDR 4500-A scanner with software version 4.84 (Waltham, MA) was used in 309 women. Owing to upper-extremity dominance, the BMD at the proximal femur was measured at non-dominant sites. The short-term in-vivo measurement precisions, expressed as the coefficient of variation, for the Lunar and Hologic machines, respectively, were 0.82 and 0.85% for the lumbar spine and 1.12 and 1.20% for the femoral neck. These values were obtained by scanning 17 volunteers who were not part of the study; each volunteer underwent five scans on the same day, getting on and off the table between examinations. To derive cross-calibration equations between the two systems, BMD values were measured at the lumbar and femoral neck by the two machines in 109 healthy Korean women (55 ± 11 years, range 31–75 years), and cross-calibration equations were calculated as follows (Jo et al. 1999):
Detection of vertebral and non-vertebral fractures
The assessment of vertebral fractures was made in accordance with the recommendations of the Working Group on Vertebral Fractures (Kiel 1995; Orimo et al. 2001). A vertebral fracture was defined quantitatively as a loss of 15% (for vertebrae that were intact at the previous examination) or a reduction of 4 mm in any of the measured vertebral dimensions (anterior, middle, or posterior) in subjects without previous history of major trauma such as a traffic accident. In addition, a history of non-vertebral fracture, such as fractures of the hip and wrist, was obtained by self-administered questionnaires from subjects who had no previous history of major trauma.
Sequencing analysis of the KIT gene
Genomic DNA was extracted from peripheral blood leukocytes using a commercial kit (Wizard Genomic DNA purification kit; Promega, Madison, WI). We sequenced the exons and their boundaries of the KIT gene, including the promoter region (≈1.5 kb), to discover genetic variants in 24 DNA samples from Korean women using a DNA analyzer (ABI PRISM 3700; Applied Biosystems, Foster City, CA). GenBank sequences were used to design 23 primer sets for the KIT gene for amplification and sequencing analysis (reference genomic sequence for KIT: accession number NT_022853) (Supplementary Table 1).
Genotyping using fluorescence polarization detection
For genotyping of three polymorphic sites, which were selected based on minor allele frequency (MAF > 0.1) and linkage disequilibrium (LD), amplifying primers and probes were designed for TaqMan (Livak 1999). Primer Express (Applied Biosystems) was used to design both the PCR primers and the MGB TaqMan probes (Supplementary Table 2). One allelic probe was labeled with FAM dye and the other with the fluorescent dye VIC. PCR was performed using TaqMan Universal Master mix without uracil DNA glycosylase (UNG) (Applied Biosystems), with PCR primer concentrations of 900 nM and TaqMan MGB-probe concentrations of 200 nM. Reactions were performed in a 384-well format in a total reaction volume of 5 μl using 20 ng genomic DNA. The plates then were placed in a thermal cycler (PE 9700, Applied Biosystems) and heated at 50°C for 2 min, then 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The TaqMan assay plates were transferred to a Prism 7900HT instrument (Applied Biosystems), and the fluorescence intensity in each well of the plate was measured. Fluorescence data files from each plate were analyzed using automated software (SDS 2.1, Applied Biosystems).
Statistics
To determine whether individual variants were in equilibrium at each locus in the population, Hardy–Weinberg equilibriums (HWE) were calculated using χ 2 tests. In case of SNPs that were genotyped only by re-sequencing (n = 24), Fisher’s exact tests for HWE (Wigginton et al. 2005) were used. We examined Lewontin’s D′ (|D′|) and the LD coefficient (r 2) between all pairs of biallelic loci. Haplotypes (ht) of each individual were inferred using the algorithm developed by Stephens et al. (2001), which uses a Bayesian approach incorporating a priori expectations of haplotype structure based on population genetics and coalescent theory. Phase probabilities of all polymorphic sites for haplotypes were calculated for each individual using this software. Individuals with phase probabilities less than 97% were excluded from the analysis. The genetic effects of inferred haplotypes were analyzed in the same way as polymorphisms. Multiple regression analyses were performed for BMD, controlling for age (continuous variable), years since menopause (YSM; continuous variable), and patient weight and height as covariates. The genotype and haplotype distributions between the subjects with and without vertebral and non-vertebral fractures were also analyzed by a logistic regression model controlling for age, YSM, weight, and height. Akaike information criterion (AIC) for all possible models were estimated to select the adequate model for this study using stepwise model selection (SAS) (Akaike 1974). The effective number of independent marker loci was calculated to correct for multiple testing, using the software SNPSpD (http://www.genepi.qimr.edu.au/general/daleN/SNPSpD/), which is based on the spectral decomposition (SpD) of matrices of pair-wise LD between SNPs (Nyholt 2004). The resulting number of independent marker loci (6.9477) was applied to correct for multiple testing.
Results
The mean age of the participants was 58.9 ± 7.5 years (range 46–83 years), and the mean YSM was 9.6 ± 7.9 years (range 1–35 years) (Table 1). BMD values measured with the Lunar equipment (0.887 ± 0.145 and 0.734 ± 0.105 g/cm2 at the lumbar spine and femoral neck, respectively) were significantly greater than those measured with the Hologic equipment (0.785 ± 0.092 and 0.608 ± 0.067 g/cm2, respectively; P < 0.0001 for both). However, the values were not significantly different after application of cross-calibration equations (data not shown). Thus, the equipment used was not included as a covariate in multiple regression analyses for associations between the BMD values and polymorphisms. As expected, age and YSM were negatively correlated with BMD, and weight was positively correlated with BMD (Table 1). Height was significantly associated with lumbar BMD.
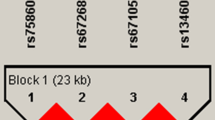
Through the direct sequencing of all exons and their boundaries of KIT, including 1,500 bp of the 5′-flanking region, 18 SNPs were identified in this study. Four were located in the promoter region, three in coding regions of exons (one non-synonymous and two synonymous), two in the 3′ untranslated regions (UTRs), and nine in the introns (Fig. 1). Pair-wise comparisons among all 18 polymorphisms revealed one set of markers in absolute LD (|D′| = 1 and r 2 = 1, Fig. 1a). Of these 18 variants, three common polymorphisms (−1694G>T, +41894A>G, and +49512G>A) were selected for larger-scale genotyping (n = 946) based on minor allele frequency (MAF > 0.1) and LD. The MAFs of the three genotyped polymorphisms were 0.446 (−1694G>T), 0.258 (+41894A>G), and 0.217 (+49512G>A) (Fig. 1a). There were no significant deviations from the HWE for any of the polymorphisms (P > 0.05; Table 2). Of the three different polymorphisms, we identified five common haplotypes (frequency > 0.05) that account for 96.1% of observed haplotypes (Fig. 1b).
Gene maps and haplotypes of KIT. Coding exons are marked by black blocks, and 5′ and 3′ UTRs are indicated by white blocks. The first base of the translational start site is denoted as nucleotide +1. Asterisks indicate the SNPs genotyped in a larger population. a Polymorphisms identified in KIT on chromosome 4q11-q12 (reference genotype sequence: NT_022853). b Haplotypes of KIT. Only those with frequencies >0.05 are shown. c Linkage disequilibrium coefficients (|D′| and r 2) among KIT polymorphisms
None of the SNPs and haplotypes was significantly associated with BMD at the lumbar spine (Supplementary Table 3). In association analysis of the BMD of the femoral neck, three SNPs of the KIT gene also showed no association; however, haplotype analyses showed that the ht3 haplotype (−1694T -+41894A -+49512G) was marginally or significantly associated with BMD values at either the lumbar spine or femoral neck in the recessive model after adjustments for covariates (P = 0.07 and 0.007, respectively; Table 3). Although only 22 of the total 946 individuals were ht3 homozygote (2.3%), the ht3 homozygote subjects had lower BMD than ht3 non-homozygote individuals. Even after correction for multiple testing, the significance in the femoral neck persisted (P = 0.05). The effects of KIT polymorphism on the risk of vertebral and non-vertebral fractures were also analyzed. Vertebral and non-vertebral fractures were noted in 126 and 107 subjects, respectively. None of the polymorphisms and haplotypes were associated with increased risk of fracture, although the ht3 haplotype tended to show increased risk of fracture in the recessive model [odds ratio (OR) = 2.21, 95% confidence interval (CI) = 0.60–8.22 for vertebral fracture, and OR = 2.07, 95% CI = 0.68–6.34 for non-vertebral fracture] (data not shown).
In addition to multiple regression analyses for all the polymorphisms with BMD of the femoral neck, AIC values for all possible subsets of multiple regression models were also estimated to select the adequate model for this study using SAS (Akaike 1974). The results of AIC revealed that the recessive model of KIT-ht3 and covariates was the most adequate model (data not shown). The genetic effect of KIT-ht3/ht3 on femoral BMD along with other covariates (height, weight, age and year since menopause) was shown in Supplementary Table 4.
Discussion
In the present study, we focused on the role of KIT polymorphisms on bone mass and the risk of osteoporotic fracture. In this study, we identified three polymorphisms of KIT in the Korean population, and none of these polymorphisms were associated with BMD. However, the ht3 homozygote subjects had significantly lower BMD at the femoral neck (P = 0.007). These results indicate that KIT-associated signaling may affect BMD. To our knowledge, this is the first clinical report of the effects of KIT on bone metabolism. However, 22 of the total of 946 subjects were ht3 homozygote (2.3%), and these subjects were shown to have BMD values about 3% lower than ht3 non-homozygote subjects. These results indicate that ht3 has only a small effect on the development of osteoporosis in the postmenopausal general population.
The ht3 haplotype, which consisted of one SNP at the putative promoter region (−1694T) and two at intronic sites (+41894G and +49512A), was significantly associated with BMD. However, no SNPs had a significant association with BMD, and the +41894A>G and +49512G>A polymorphisms are located in introns and do not cause an amino acid change. Therefore, for now, it seems sensible to interpret ht3 as a marker rather than a direct contributor to genetic function. However, it has been speculated that the −1694 site is a putative C/EBPα-binding site. C/EBPα has a significant role in hematopoietic differentiation (Cammenga et al. 2003), which indicates that −1694G>T may affect promoter activity due to changes to the C/EBPα binding site. Furthermore, it has been suggested that intronic polymorphisms may affect genetic function via the change in alternative splicing (Ast 2004). Therefore, the combined effects of these genetic variations may have a functional relevance.
There were several possible limitations to this study. First, the study population comprised women who visited a university hospital and who may not be representative of the general population residing in a community, thereby resulting in possible selection bias. Second, despite the significant associations of ht3 with lower BMD at the femoral neck, the ht3 was not associated with increased risk of osteoporotic fracture. This indicates that many environmental factors other than BMD play important roles in determining the fracture risk. In fact, the heritability of fracture risk has been estimated as 25–35% (Deng et al. 2000; MacGregor et al. 2000), which is much lower than the heritability of the BMD values (Eisman 1999; Peacock et al. 2002). Therefore, the fact that there was no association between the ht3 and fracture risk cannot exclude a possible role of ht3 as a genetic marker for bone metabolism. Therefore, it might be worthwhile to follow up on the signals of this gene through larger cohort studies.
In summary, to examine the possible involvement of genetic polymorphism of KIT in osteoporosis, three polymorphisms in KIT were identified and genotyped in postmenopausal Korean women (n = 946). Logistic and regression analyses found genetic linkage of ht3 with reduced BMD in Korean postmenopausal women. These data suggest that the KIT gene is likely to contribute, in part, to genetic susceptibility to osteoporosis in postmenopausal women.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Autom Control 19:716–723
Arden NK, Spector TD (1997) Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res 12:2076–2081
Ast G (2004) How did alternative splicing evolve? Nat Rev Genet 5:773–782
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Cammenga J, Mulloy JC, Berguido FJ, MacGrogan D, Viale A, Nimer SD (2003) Induction of C/EBPalpha activity alters gene expression and differentiation of human CD34+ cells. Blood 101:2206–2214
Demulder A, Suggs SV, Zsebo KM, Scarcez T, Roodman GD (1992) Effects of stem cell factor on osteoclast-like cell formation in long-term human marrow cultures. J Bone Miner Res 7:1337–1344
Deng HW, Chen WM, Recker S, Stegman MR, Li JL, Davies KM, Zhou Y, Deng H, Heaney R, Recker RR (2000) Genetic determination of Colles’ fracture and differential bone mass in women with and without Colles’ fracture. J Bone Miner Res 15:1243–1252
Eisman JA (1999) Genetics of osteoporosis. Endocr Rev 20:788–804
Gattei V, Aldinucci D, Quinn JM, Degan M, Cozzi M, Perin V, Iuliis AD, Juzbasic S, Improta S, Athanasou NA, Ashman LK, Pinto A (1996) Human osteoclasts and preosteoclast cells (FLG 29.1) express functional c-kit receptors and interact with osteoblast and stromal cells via membrane-bound stem cell factor. Cell Growth Differ 7:753–763
Grano M, Galimi F, Zambonin G, Colucci S, Cottone E, Zallone AZ, Comoglio PM (1996) Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci USA 93:7644–7648
Jo JK, Kim JS, Kim GS, Kim SW, Shin JW, Moon DH, Lee HK (1999) Cross-calibration of bone mineral density between two different dual X-ray absorptiometry system: hologic QDR 4500-A and lunar EXPERT-XL. Korean J Nucl Med 33:282–288
Kiel D (1995) Assessing vertebral fractures. National Osteoporosis Foundation Working Group on vertebral fractures. J Bone Miner Res 10:518–523
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Lean JM, Fuller K, Chambers TJ (2001) FLT3 ligand can substitute for macrophage colony-stimulating factor in support of osteoclast differentiation and function. Blood 98:2707–2713
Livak KJ (1999) Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal 14:143–149
Lowry PA, Deacon D, Whitefield P, McGrath HE, Quesenberry PJ (1992) Stem cell factor induction of in vitro murine hematopoietic colony formation by “subliminal” cytokine combinations: the role of “anchor factors”. Blood 80:663–669
MacGregor A, Snieder H, Spector TD (2000) Genetic factors and osteoporotic fractures in elderly people. Twin data support genetic contribution to risk of fracture. BMJ 320:1669–1670
Nathan M, Pope MH, Grobler LJ (1994) Osteophyte formation in the vertebral column: a review of the etiologic factors—Part II. Contemp Orthop 29:113–119
Nyholt DR (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74:765–769
Orimo H, Hayashi Y, Fukunaga M, Sone T, Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J, Oh-Hashi Y, Hosoi T, Gorai I, Tanaka H, Igai T, Kishimoto H (2001) Diagnostic criteria for primary osteoporosis: year 2000 revision. J Bone Miner Metab 19:331–337
Peacock M, Turner CH, Econs MJ, Foroud T (2002) Genetics of osteoporosis. Endocr Rev 23:303–326
Silberstein R, Melnick M, Greenberg G, Minkin C (1991) Bone remodeling in W/Wv mast cell deficient mice. Bone 12:227–236
Slemenda CW, Christian JC, Williams CJ, Norton JA, Johnston CC Jr (1991) Genetic determinants of bone mass in adult women: a reevaluation of the twin model and the potential importance of gene interaction on heritability estimates. J Bone Miner Res 6:561–567
Stephens M, Smith NJ, Donnelly P (2001) A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68:978–989
Suda T, Nakamura I, Jimi E, Takahashi N (1997) Regulation of osteoclast function. J Bone Miner Res 12:869–879
van’t Hof RJ, von Lindern M, Nijweide PJ, Beug H (1997) Stem cell factor stimulates chicken osteoclast activity in vitro. FASEB J 11:287–293
Wigginton JE, Cutler DJ, Abecasis GR (2005) A note on exact tests of Hardy–Weinberg equilibrium. Am J Hum Genet 76:887–893
Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A (1987) Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J 6:3341–3351
Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, Hsu RY, Birkett NC, Okino KH, Murdock DC et al (1990) Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell 63:213–224
Acknowledgments
This work was supported by a grant from the Korea Health 21 R & D Project, Ministry of Health and Welfare, Republic of Korea (Project 01-PJ3-PG6-01GN111-0002).
Author information
Authors and Affiliations
Corresponding author
Additional information
S.-Y. Kim and J.-Y. Lee are co-first authors.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Kim, SY., Lee, JY., Kim, H.Y. et al. Association of KIT gene polymorphisms with bone mineral density in postmenopausal Korean women. J Hum Genet 52, 502–509 (2007). https://doi.org/10.1007/s10038-007-0143-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-007-0143-4