Abstract
We showed that humanin (HN), an endogenous peptide against Alzheimer disease-related insults, was expressed in muscles of patients with chronic progressive external ophthalmoplegia (CPEO), a major mitochondrial disease. Because HN was recently found to block proapoptotic Bax function and exert its versatile cytoprotective effects in association with an increase in ATP levels, HN expression may thus reflect a physiological response against degenerative changes in the muscles of patients with CPEO. We found HN expression in all four patients examined, each of whom had different mitochondrial DNA mutations including two different single DNA deletions, multiple deletions, and no major mutations detected. We also found that HN expression was not linked to focal cytochrome c deficiency, strongly associated with the subtype of CPEO with single deletions. These results suggest that HN expression is more closely related to degenerative changes in all types of CPEO. Notably, HN was also expressed in non-degenerative muscle fibers of patients with CPEO or Leigh syndrome, who had the 8993T>G mutation in the mitochondrial ATPase 6 gene known to be associated with impaired ATP synthesis. Collectively, our findings suggest that HN may be specifically expressed in response to defects in energy production in muscles with mitochondrial abnormalities.
Similar content being viewed by others
Introduction
Chronic progressive external ophthalmoplegia (CPEO) is associated mainly with mitochondrial DNA (mtDNA) abnormalities (Lee and Brazis 2002). About 60% of patients with CPEO have single large deletions in mtDNA, while the others have multiple deletions, a 3243A>G point mutation, or no detectable major mutations (Lee and Brazis 2002). Despite different genetic backgrounds, the similar clinical and pathological manifestations suggest a common mechanism for the pathogenesis of CPEO, which may include defects in mitochondrial energy production or increases in reactive oxygen species (Wallace 2000). These mechanisms also play a role in other mitochondrial diseases (Brown and Wallace 1994). We have recently reported that skeletal muscles in patients with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) express humanin (HN), an endogenous peptide that increases cellular ATP (Kariya et al. 2005a). Although HN is expressed mainly in ragged-red fibers (RRFs), its expression is not a result of mitochondrial proliferation, since HN rather decreased pathological increases in mtDNA in vitro (Kariya et al. 2005b). These findings suggest that HN may be expressed in affected muscles to cope with defects in mitochondrial energy production in MELAS (Kariya et al. 2005b). To further explore this hypothesis, we investigated the expression of HN in muscles from patients who had CPEO with different mitochondrial mutations. We also analyzed muscles from patients who had Leigh syndrome (LS) with 8993T>G mutations in the mitochondrial ATPase 6 gene, known to be associated with impaired ATP synthesis.
Materials and methods
Patients
The clinical and genetic information on four patients with CPEO and two patients with LS is summarized in Table 1. Mitochondrial disease was diagnosed by means of clinical, laboratory, histological, histochemical, biochemical, and genetic investigations according to generally accepted criteria and methods (Morgan-Hughes 1994). While other patients had no family history, patient 3 has an affected relative (a daughter of father’s sister), suggesting an autosomal dominant inheritance with incomplete penetrance. Multiple deletions in mtDNA may result from nuclear DNA abnormalities, such as mutations in the ANT1, POLG1, and Twinkle genes (Filosto et al. 2003) in autosomal disorders, although the mutation in the causative autosomal genes in this patient remains unknown. Muscle biopsy and analysis of mtDNA in muscle tissue were performed as described previously (Goto et al. 1992; Akanuma et al. 2000; Kariya et al. 2005b), after obtaining informed consent from the patients or their guardians.
Histological analysis
All specimens were obtained from biceps brachii muscles and quickly frozen in liquid nitrogen-cooled isopentane. Frozen serial transverse sections from four patients with CPEO and two patients with LS were stained with modified Gomori trichrome, succinate dehydrogenase (SDH), and cytochrome c oxidase (COX). The sections were also processed for immunostaining with a rabbit polyclonal anti-HN antibody (dilution 1:200, TransGenic, Kumamoto, Japan). In addition, frozen muscle sections obtained from two patients with Duchenne muscular dystrophy (DMD) were analyzed immunohistochemically. As a normal control, we analyzed muscle specimens obtained from three patients who underwent muscle biopsies to diagnose the cause of neuromuscular symptoms, but were later confirmed to have no abnormalities on clinical, electrophysiological, or histological examinations. Normal mouse IgG, diluted to the same concentration as the primary antibodies, was used as a negative control. All muscle samples were examined under a fluorescent microscope (Axiophot2, Zeiss, Göttingen, Germany).
Results
Results of genetic analysis
Southern blot and sequencing of mtDNA revealed a single 4,590-bp deletion [nucleotides (nt) 10,940–15,529] with 5-bp direct repeats at the break points in patient 1, and a 7,663-bp deletion (nt 6,342–14,004) with 10-bp direct repeats at the break points in patient 2 (data not shown). The deleted part of DNA in patient 2 encodes all three mitochondrial subunits of COX, while that in patient 1 does not encode any of these subunits. Southern blot and long PCR analyses showed that patient 3 had multiple deletions in their mtDNA (Fig. 1). Patient 4 had no deletions or major point mutations, including 3243A>G, 3271T>C, 8344A>G, 8356T>C, or 8363G>A mutations. Patients 5 and 6 (LS) had essentially homoplasmic 8993T>G mutations in mitochondrial DNA (data not shown).
Expression of endogenous HN in CPEO muscles
Representative findings from patients 1 and 3 are shown in Fig. 2. HN was strongly positive in all RRFs and mildly positive in some non-RRFs of all patients examined. The number of HN-positive non-RRFs is summarized in Table 1. The remaining non-RRFs showed a background level of HN expression. HN immunoreactivity in control or DMD samples treated with both first and secondary antibodies was slightly stronger than that in samples treated with only a secondary antibody, indicating that endogenous HN was marginally expressed in normal control fibers (not shown) (Kariya et al. 2005b). However, the expression level was much lower than that in CPEO muscles. This study detected no apparent strongly SDH reactive blood vessels in CPEO muscles, consistent with the results of previous studies (Goto 1995); consequently, no HN-positive vessels were observed.
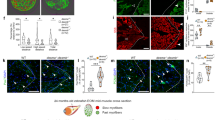
Expression of endogenous humanin (HN) in muscle specimens from patients with chronic external ophthalmoplegia (CPEO) and Leigh syndrome (LS). a, e, g, k, m Modified Gomori trichrome; b, h, n succinate dehydrogenase (SDH); c, i, o cytochrome c oxidase (COX); d, f, j, l, p HN. A goat anti-rabbit FITC-conjugated IgG antibody was used to visualize HN. a–d, e–f Patient 1 (single mtDNA deletion); g–j, k–l patient 3 (multiple deletions), m–p patient 5 (LS) are transverse serial sections. Muscle specimens from patient 1 showed a COX-negative ragged-red fiber (RRF), while those from patient 3 showed a COX-positive RRF. HN was strongly positive in all RRFs (asterisks in panels d and j) and mildly positive in some non-RRFs (asterisks in panels f, l, and p )
Expression of endogenous HN in muscles from LS
Alhough muscles from LS had no RRFs, some non-RRFs were mildly positive for HN. Representative findings from patients 5 are shown in Fig. 2. The number of HN-positive non-RRFs is summarized in Table 1.
COX and HN expression in CPEO
Focal COX deficiency was observed in all muscle specimens from patients with CPEO, regardless of the type of mitochondrial mutation and the deletion site. All RRFs in CPEO associated with single deletions were negative for COX, irrespective of deletion sites containing mitochondrial COX subunits, as reported previously (Moraes et al. 1992). Similarly, patient 4, who had no major mutations, showed COX-negative RRFs. By contrast, all RRFs in CPEO with multiple deletions were strongly positive for COX. Serial section analyses showed that both COX-positive and -negative RRFs were strongly positive for HN (Fig. 2).
Discussion
This study showed, for the first time to our knowledge, high HN expression in all RRFs and mild expression in some non-RRFs of all patients with CPEO examined. Given that HN increases ATP levels in vitro (Kariya et al. 2005b), our results suggest that defects in energy production by affected muscle fibers may be initially compensated for by HN, whereas further progressive defects may ultimately lead to degenerative RRFs (Kariya et al. 2005b). Although its precise physiological role remains unknown, HN was originally identified as an endogenous peptide against Alzheimer disease-related insults (Hashimoto et al. 2001) and was found to block proapoptotic Bax function and exert its versatile cytoprotective effects in association with an increase in ATP levels (Hashimoto et al. 2001; Guo et al. 2003; Kariya et al. 2003, 2005b). HN expression may thus reflect a physiological response against degenerative changes in the muscles of patients with CPEO.
HN expression was found in patients with CPEO, regardless of the type of mitochondrial mutation. To support the association of HN expression with the common pathological pathway in CPEO, especially with ATP deficiency, we analyzed muscles from two patients with LS who had essentially homoplasmic 8993T>G mutations in the mitochondrial ATPase 6 gene, which are known to be associated with impaired ATP synthesis (Sgarbi et al. 2006). Although patients with LS generally lack RRFs similar to our patients (Uziel et al. 1997), mildly increased HN expression was observed in some non-RRFs of both patients. This finding suggests that HN expression is not necessarily associated with RRFs, but may be associated with ATP deficiency. However, because HN expression was not increased in many muscle fibers, factors other than ATP levels, including ATP demand in muscle fibers, may determine HN expression.
One major reported difference between MELAS and CPEO is distinct COX expression levels in RRFs: COX expression levels range from low to high in MELAS or CPEO with a 3243A>G mutation, but are nil or minimal in CPEO associated with single deletions (Goto et al. 1990). Our study similarly showed that COX expression was negative in muscles associated with single deletions or no major mutations. In addition, we found that COX expression levels were high in the muscles of a patient with multiple deletions, a finding not reported previously. In this patient, COX deficiency may not have been primarily responsible for defective energy production. This remains speculative, however, because we studied only a single patient with multiple deletions; confirmation must await further studies. In contrast to different COX levels in RRFs, HN was expressed in all RRFs, indicating that COX expression and HN expression were independently regulated. This finding also suggests that HN expression is more closely related to degenerative changes in all types of CPEO. In contrast, COX expression appears to be more strongly associated with the subtype of CPEO with single deletions, since in this subtype the frequency of focal COX deficiency is significantly linked to an increased ratio of deleted mtDNA (Goto et al. 1990).
In summary, we found that HN was expressed in the muscles of patients with CPEO, independently of mtDNA deletions and of COX expression. However, HN expression was apparently not nonspecific because detectable expression levels of HN were marginal in normal controls and patients with DMD. Although our present and previous studies did not examine muscles affected by mitochondrial diseases other than CPEO, MELAS, and LS, our findings suggest that HN may be specifically expressed in response to defects in energy production in muscles with mitochondrial abnormalities.
References
Akanuma J, Muraki K, Komaki H, Nonaka I, Goto Y (2000) Two pathogenic point mutations exist in the authentic mitochondrial genome, not in the nuclear pseudogene. J Hum Genet 45:337–341
Brown MD, Wallace DC (1994) Molecular basis of mitochondrial DNA disease. J Bioenerg Biomembr 26:273–289
Filosto M, Mancuso M, Nishigaki Y, Pancrudo J, Harati Y, Gooch C, Mankodi A, Bayne L, Bonilla E, Shanske S, Hirano M, DiMauro S (2003) Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol 60:1279–1284
Goto Y (1995) Clinical features of MELAS and mitochondrial DNA mutations. Muscle Nerve 3:S107–S112
Goto Y, Koga Y, Horai S, Nonaka I (1990) Chronic progressive external ophthalmoplegia: a correlative study of mitochondrial DNA deletions and their phenotypic expression in muscle biopsies. J Neurol Sci 100:63–69
Goto Y, Horai S, Matsuoka T, Koga Y, Nihei K, Kobayashi M, Nonaka I (1992) Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA. Neurology 42:545–550
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC (2003) Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 423:456–461
Hashimoto Y, Niikura T, Tajima H, Yasukawa T, Sudo H, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I (2001) A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci USA 98:6336–6341
Kariya S, Takahashi N, Hirano M, Ueno S (2003) Humanin improves impaired metabolic activity and prolongs survival of serum-deprived human lymphocytes. Mol Cell Biochem 254:83–89
Kariya S, Hirano M, Furiya Y, Ueno S (2005a) Effect of humanin on decreased ATP levels of human lymphocytes harboring 3243A>G mutant mitochondrial DNA. Neuropeptides 39:97–101
Kariya S, Hirano M, Furiya Y, Sugie K, Ueno S (2005b) Humanin detected in skeletal muscles of MELAS patients: a possible new therapeutic agent. Acta Neuropathol 109:367–372
Lee AG, Brazis PW (2002) Chronic progressive external ophthalmoplegia. Curr Neurol Neurosci Rep 2:413–417
Moraes CT, Ricci E, Petruzzella V, Shanske S, DiMauro S, Schon EA, Bonilla E (1992) Molecular analysis of the muscle pathology associated with mitochondrial DNA deletions. Nat Genet 1:359–367
Morgan-Hughes JA (1994) Mitochondrial diseases. In: Engel AG, Franzini-Armstrong C (eds) Myology. 2nd edn. McGraw-Hill, New York pp 1610–1660
Sgarbi G, Baracca A, Lenaz G, Valentino LM, Carelli V, Solaini G (2006) Inefficient coupling between proton transport and ATP synthesis may be the pathogenic mechanism for NARP/Leigh syndromes resulting from the 8993T>G mutation in mtDNA. Biochem J [Epub ahead of print] https://doi.org/10.1042/BJ20051748
Uziel G, Moroni I, Lamantea E, Fratta GM, Ciceri E, Carrara F, Zeviani M (1997) Mitochondrial disease associated with the T8993G mutation of the mitochondrial ATPase 6 gene: a clinical, biochemical, and molecular study in six families. J Neurol Neurosurg Psychiatry 63:16–22
Wallace DC (2000) Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am Heart J 139:S70–S85
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kin, T., Sugie, K., Hirano, M. et al. Humanin expression in skeletal muscles of patients with chronic progressive external ophthalmoplegia. J Hum Genet 51, 555–558 (2006). https://doi.org/10.1007/s10038-006-0397-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-006-0397-2
Keywords
This article is cited by
-
Oxidative Damage? Not a Problem! The Characterization of Humanin-like Mitochondrial Peptide in Anoxia Tolerant Freshwater Turtles
The Protein Journal (2021)
-
Humanin Promotes Tumor Progression in Experimental Triple Negative Breast Cancer
Scientific Reports (2020)
-
Sex differences in the late first trimester human placenta transcriptome
Biology of Sex Differences (2018)
-
Protective Effects of Humanin and Calmodulin-Like Skin Protein in Alzheimer’s Disease and Broad Range of Abnormalities
Molecular Neurobiology (2015)
-
Secreted calmodulin-like skin protein inhibits neuronal death in cell-based Alzheimer’s disease models via the heterotrimeric Humanin receptor
Cell Death & Disease (2013)