Abstract
COL7A1 glycine substitution (GS) mutations result in dominant and recessive dystrophic epidermolysis bullosa (DDEB and RDEB). Here, we report a DDEB family in which retention of type VII collagen by epidermal keratinocytes was observed for a female proband. Mutational analysis detected a GS mutation, G2037E, in the proband and her affected father. To demonstrate direct association of G2037E and type VII collagen retention we introduced this mutated COL7A1 gene into cultured keratinocytes using retroviral methods. This mutation was dominant, so we transferred a 1:1 mixture of wild-type (unaffected) and G2037E-mutated COL7A1, together, in addition to the unaffected gene or the mutated gene alone. The increase in type VII collagen cytoplasmic staining in the G2037E/wild transfectant cell samples was compared with that for control/wild-type cells. Intracellular collagen VII staining in the G2037E (alone)-transfected cells was even stronger than for the G2037E/wild transfection sample. These results indicate that the G2037E COL7A1 mutation leads to increased epidermal retention of type VII collagen in vivo, and also suggests that homozygotes carrying this dominant GS mutation may have more severe phenotypes than heterozygotes. This study furthers our understanding of GS COL7A1 mutations in DEB.
Similar content being viewed by others
Introduction
Type VII collagen, a non-fibrillar collagen, is a major component of anchoring fibril loop structures beneath the epidermal basement membrane (Uitto et al. 1992; Burgeson 1993). Mutations within the type VII collagen gene (COL7A1) are associated with dystrophic forms of epidermolysis bullosa (DEB) (Christiano et al. 1993). DEB is clinically characterized by mucocutaneous blistering in response to minor trauma, followed by scarring and nail dystrophy in which tissue dermal–epidermal separation is observed beneath the lamina densa at the level of the anchoring fibrils. It is inherited in either an autosomal dominant (DDEB) or recessive (RDEB) fashion, each form having a specific, slightly different clinical presentation and severity (Fine et al. 2000). Study of DEB mutations has revealed several general genotype–phenotype correlations (Pulkkinen and Uitto 1999).
Recessive dystrophic epidermolysis bullosa patients may harbor any type of COL7A1 mutation including premature termination codons, missense, glycine substitution (GS), or splice site mutations on both alleles. GS mutations on one allele have been found in many DDEB patients and in-frame deletion mutations have been observed for a few patients. Thus, COL7A1 GS mutations can cause both DDEB and RDEB subtypes (Christiano et al. 1995; Shimizu et al. 1996).
During the course of COL7A1 DEB patient mutational analysis (Sawamura et al. 2005) we found a unique GS mutation which was associated with retention of type VII collagen in keratinocytes. Some, but not all, GS COL7A1 mutations result in intracellular accumulation of collagen VII (Hammami-Hauasli et al. 1998; Shimizu et al. 1999). To obtain direct evidence of whether G2037E leads to intracytoplasmic retention of type VII collagen we introduced the mutated COL7A1 gene into cultured keratinocytes.
Materials and methods
Patient
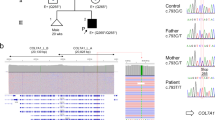
A Japanese girl presented with erosions and blisters affecting her trunk and lower extremities that had persisted since birth. The blisters continued to appear, particularly at sites of trauma. Physical examination revealed bullae on her hands, feet, and abdomen (Fig. 1B). Healing occurred with minimal scarring and occasional milia formation. Her father had a similar history, and blister formation and the resulting scars were apparent, predominantly on the knees and elbows (Fig. 1C). A family tree is shown in Fig. 1A. Informed consent to the study and to publication of the clinical images were obtained from the family.
Ultrastructural and immunohistochemical studies
Skin biopsies were taken from the affected child, and processed for transmission electron microscopy and immunofluorescence microscopy, as described elsewhere (Shimizu et al. 1996). For ultrastructural examination, skin specimens were fixed in 5% glutaraldehyde, postfixed in 1% osmium tetroxide, and stained en-block in uranyl acetate. They were dehydrated in a graded series of ethanol solutions and then embedded in Araldite 6005. Ultrathin sections were cut and stained with uranyl acetate and lead citrate. The sections were examined with a transmission electron microscope (H-7100; Hitachi, Tokyo, Japan) at 75 kV. For immunohistochemical examination the specimens were embedded in OCT compound and 5 μm thick sections were cut. The anti-human type VII collagen monoclonal antibody (LH7.2: kind gift from I. Leigh, UK) directed against the NC-1 amino terminal domain of the protein was used for experiments. The bound antibodies were detected with FITC-conjugated goat anti-mouse IgG antibody.
Mutational analysis
Genomic DNA was isolated from peripheral blood lymphocytes of patients and their families, by using standard procedures. COL7A1 segments including all 118 exons, all exon–intron borders, and the promoter region were amplified by PCR using pairs of oligonucleotide primers synthesized on the basis of intronic sequences in accordance with the report by Christiano et al. (1997) (GenBank numbers L02870, L23982). Specifically, to amplify exons 73 the primers used were: sense primer 5′-aagtggctcagtgggttgtg-3′; antisense primer 5′-aacccctcttccctcactct-3′. For PCR amplification, approximately 200 ng genomic DNA, 40 pmol of each primer, 0.5 mmol−1 MgCl2, 20 μmol of each dNTP, and 1.25 U Taq polymerase were used in a total volume of 50 μL. The amplification conditions were 94°C for 5 min, then 40 cycles of 94°C for 45 s, 55–60°C for 45 s, 72°C for 45 s, and extension at 72°C for 10 min in GeneAmp PCR System 9700 (Applied Biosystems, Foster City, CA, USA). The PCR products were subjected to direct automated nucleotide sequencing using the BigDye Terminator System (Applied Biosystems).
Construction of retroviral COL7A1 expression vectors and transfection
Normal human full-length COL7A1 cDNA was constructed from several overlapping cDNA clones (Sawamura et al. 2002). COL7A1 mutations 2037E, 6110G>A, and G2043R; 6127G>A were generated by an in-vitro mutagenesis technique using a Mutant-Super Express Km Kit (Takara, Otsu, Shiga, Japan). A retroviral vector pDON(Δ) was constructed by removing the SV-40 promoter and Neo gene from pDON-AI (Takara) and both the wild and mutated full-length COL7A1 cDNAs were inserted into pDON(Δ) (Goto et al. 2006). The recombinant retroviruses were produced by transfecting the retroviral plasmids into the amphotropic amphopack-293 packaging cells (Clontech, Mountain View, CA, USA) using a calcium–phosphate co-precipitation method. We also used the G protein of the vesicular stomatitis virus (VSV-G), a pseudotyped retrovirus vector (Clontech). The retroviral plasmids and plasmid pVSV-G were cotransfected into pantropic GP2-293 packaging cells (Clontech). We applied the mutated gene, wild-type (normal) COL7A1 gene (control), and a 1:1 mixture of mutated and normal genes. The viral particles were recovered from the cell culture medium and ultracentrifugation was performed for concentration of viruses with both normal and mutated COL7A1 constructs.
Expression of mutated type VII collagen
The HaCaT human keratinocyte cell line was maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. HaCaT cells were expanded to 60% of confluent density and then transduced with viral suspensions in 5 μg mL−1 polybrene. To increase attachment of virus to keratinocytes, we coated the surface of culture plates with 10 ng mL−1 retronectin (Takara; fibronectin fragment CH-296). After incubation for 24 h at 32°C we maintained the treated keratinocytes with fresh medium for another 72 h and immunostaining was performed using the monoclonal antibody LH7.2. Digital images were analyzed on an Apple G5 computer (Apple, Cupertino, CA, USA) using the public domain NIH Image program (developed at the National Institutes of Health and available on the Internet at http://www.rsb.info.nih.gov/nih-image/). To semiquantify COL7A1 expression the HaCaT cells were classified into low, medium, and high expression depending on pixel values. We evaluated 100 fluorescing cells and the expression index value was calculated by use of the formula: (low expression cell number)×1+(medium expression cell number)×2+(high expression cell number)×3. The expression index is shown with the mean±SD of the expression values from five different areas.
Results
Diagnosis of dominant dystrophic epidermolysis bullosa
The proband and her father had suffered from skin fragility since birth. The severity of the father’s skin lesions had improved with age and healing had occurred but with scarring. Routine ultrastructural examination showed skin separation occurred within the sublamina densa in the region of the anchoring fibrils (Fig. 2A), suggesting DEB. The number of anchoring fibrils was also reduced. Immunofluorescence study using LH7.2 detected a linear staining pattern in the basement membrane zone, which was not characteristic of HS-RDEB (Fig. 2B). We also observed retention of type VII collagen within epidermal keratinocytes in this patient (Fig. 2B). This pattern is a characteristic feature of DDEB and transient bullous dermolysis of the newborn; this is a rare form of dystrophic epidermolysis bullosa and also caused by COL7A1 mutations (Fassihi et al. 2005). Patients with transient bullous dermolysis of the newborn present with neonatal skin blistering which usually improves markedly during early life or even remits completely. Because blister formation on this patient continued until approximately 2 years of age and her father’s skin was still fragile, we opted for diagnosis of DDEB rather than transient bullous dermolysis of the newborn.
Ultrastructural, immunohistochemical, and mutational analyses of the proband. A Ultrastructural examination showed that skin separation occurred beneath the lamina densa (★) and there were reduced numbers of anchoring fibrils. B and C Immunofluorescence study using monoclonal antibody against type VII collagen (LH7.2) detected a linear staining pattern along the basement membrane zone and retention of type VII collagen within epidermal keratinocytes (arrows) (B). Normal control individual collagen VII staining (C). D Mutational analysis of COL7A1 revealed a heterozygous G to A transition at nucleotide position 6127 in the mutant allele converting a glycine to glutamic acid (G2037E)
Mutational analysis of COL7A1 revealed a heterozygous G to A transition at nucleotide position 6110 in the mutant allele converting a glycine to glutamic acid (G2037E) (Fig. 2D). This mutation was not found in the unaffected family members. This mutation was confirmed by restriction enzyme digestion (data not shown). Thus, final diagnosis of DDEB was made on the basis of clinical and laboratory findings.
Transfection study
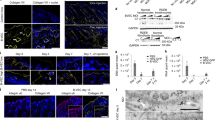
We next constructed retroviral expression vectors with mutations G2037E or G2043R as control, introduced them to keratinocytes and examined type VII collagen expression. In the G2043R transfection experiment we failed to find significantly different COL7A1 staining among the G2043R-, wild-, and G2043R/wild-treated sample groups. Semiquantitative analysis gave a similar result (Fig. 3). In contrast, we detected more intracytomic type VII collagen staining in the G2037E/wild sample than in the control wild-type sample. Intracellular collagen VII staining was also stronger in the G2037E-transfected sample than in the G2037E/wild transfection group (Fig. 3). This finding was confirmed by semiquantitative analysis, which revealed the expression indices of G2037E and G2037E/wild samples were 2.2- and 1.6-fold, respectively, higher than that of wild samples when compared with wild-type transfected controls (Fig. 3).
The effect of the glycine substitution mutation on type VII collagen retention. We constructed COL7A1 retroviral vectors with G2043R or G2037E mutations and introduced these genes into HaCaT cells. We transferred a 1:1 mixture of wild-type (normal) and mutated COL7A1 and the wild-type gene alone or the mutated gene alone. A Type VII collagen staining showed that intracellular immunoreactivity was high, in the order wild-type control samples (a: W), G2037E/wild samples (b: W/G2037E), and G2037E samples (c: G2037E). B These findings were confirmed by semiquantitative analysis, which showed the G2037E and G2037E/wild samples expression indices were higher than that of the wild samples by 2.2- and 1.6-fold, respectively. The G2043R mutation, failed to show a significant difference between the G2043R, wild, and G2043R/wild samples in COL7A1 staining. The dotted cross symbol indicates P<0.01 between W and W/G2037E, and between W/G2037E and G2037E
Discussion
Some, but not all, dominant GS mutations in COL7A1 result in intracellular accumulation of collagen VII (Hammami-Hauasli et al. 1998; Shimizu et al. 1999). The G2037E mutation has previously been reported to induce type VII collagen retention in epidermal keratinocytes (Jonkman et al. 1999). No transfection study was, however, used to demonstrate the direct relevance of dominant GS mutations to increased intracellular type VII collagen retention, although transfection studies had been performed characterizing the recessive GS G2008R mutation (Chen et al. 2002). We therefore constructed COL7A1 retroviral vectors with G2037E or G2043R mutations, and transferred these genes into HaCaT cells. The reasons we selected the G2043R mutation as a control were that this defect is a known, recurrent DDEB mutation (Mellerio et al. 1998; Wessagowit et al. 2001), and that it was the closest to the dominant substitution G2037E mutation observed in our patient. The transfection efficacy of our retroviral system was almost 30% in HaCaT cells (Goto et al. 2006). Because little or no intrinsic intracellular collagen VII expression is observed in HaCaT cells, we predicted that any high-level COL7A1-expressing cells were likely to be successfully gene-transfected cells. Those mutations were dominant, so we also transferred a 1:1 mixture of wild and mutated COL7A1 and the wild-type COL7A1 gene alone or the mutated gene alone. Transfection of G2037E mutation induced accumulation of type VII collagen in keratinocytes, whereas transfection of G2043R led to no abnormal findings. This proves that COL7A1 mutation G2037E causes epidermal retention of type VII collagen.
Glycine residues within the collagenous domain are critical for proper triple-helix formation. Some COL7A1 GS mutations, which cause RDEB in patients harboring a second mutation on the remaining allele, are silent in patients with one normal COL7A1 allele. In addition, heterozygous GS mutations can cause DDEB by dominant negative interference with the collagen triple helix. The following theoretical explanation is proposed. These dominant mutations may mildly interfere with α-chain polypeptide structure and enable the formation of abnormal triple helix structures affecting the other, normal, α-chains. The change from glycine to the mutated residue is thus thought to result in disruption or destruction of the normal triple helical structure in a dominant, negative, manner. Conversely, the recessive GS mutations are thought to completely inhibit formation of the α-chain so the mutated polypeptide cannot induce this dominant negative effect in the normal chains. As far as we know, RDEB cases homozygous for certain DDEB GS mutations have not yet been identified. In fact, heterozygous dominant GS mutations in COL4A4 can cause Alport syndrome, whereas one healthy individual is homozygous with these mutations (Boye et al. 1998). Also, in cases of COL1A2 GS mutations, clinical and laboratory findings of the heterozygote was not significantly different from those of the homozygote (DePaepe et al. 1997). Thus, it is possible that DDEB GS homozygotes may not demonstrate any significant EB phenotype.
We used the wild-type (normal) COL7A1 gene alone (control), a 1:1 mixture of mutated (diseased) and wild-type genes, and the mutated gene only (positive control). We failed to find significantly different collagen VII staining for the G2043R, wild, and G2043R/wild treatments. This mutation was not predicted to affect secretion but homotrimer formation. Our results demonstrated, however, that the G2037E mutation alone significantly affected collagen formation and this was more impaired than for the combination G2037E/wild-type gene transfected sample. This result indicates the phenotype may be more severe for homozygotes with the dominant GS mutation than for heterozygotes, suggesting the dominant GS mutation causes interference with the α-chain polypeptide structure itself and has a dominant negative effect on the collagen triple helix.
Abbreviations
- DDEB:
-
Dominant dystrophic epidermolysis bullosa
- DEB:
-
Dystrophic epidermolysis bullosa
- GS:
-
Glycine substitution
- HS:
-
Hallopeau–Siemens type
- n-HS:
-
non-Hallopeau–Siemens type
- RDEB:
-
Recessive dystrophic epidermolysis
References
Boye E, Mollet G, Forestier L, Cohen-Solal L, Heidet L, Cochat P, Grunfeld JP, Palcoux JB, Gubler MC, Antignac C (1998) Determination of the genomic structure of the COL4A4 gene and of novel mutations causing autosomal recessive Alport syndrome. Am J Hum Genet 63:329–340
Burgeson RE (1993) Type VII collagen, anchoring fibrils, and epidermolysis bullosa. J Invest Dermatol 101:252–255
Chen M, Costa FK, Lindvay CR, Han YP, Woodley DT (2002) The recombinant expression of full-length type VII collagen and characterization of molecular mechanisms underlying dystrophic epidermolysis bullosa. J Biol Chem 277:2118–2124
Christiano AM, Greenspan DS, Hoffman GG, Zhang X, Tamai Y, Lin AN, Dietz HC, Hovnanian A, Uitto J (1993) A missense mutation in type VII collagen in two affected siblings with recessive dystrophic epidermolysis bullosa. Nat Genet 4:62–66
Christiano AM, Morricone A, Paradisi M, Angelo C, Mazzanti C, Cavalieri R, Uitto J (1995) A glycine-to-arginine substitution in the triple-helical domain of type VII collagen in a family with dominant dystrophic epidermolysis bullosa. J Invest Dermatol 104:438–440
Christiano AM, Hoffman GG, Zhang X, Xu Y, Tamai Y, Greenspan DS, Uitto J (1997) Strategy for identification of sequence variants in COL7A1, and a novel 2 bp deletion mutation in recessive dystrophic epidermolysis bullosa. Hum Mutat 10:408–414
DePaepe A, Nuytinck L, Raes M, Fryns JP (1997) Homozygosity by descent for a COL1A2 mutation in two sibs with severe osteogenesis imperfecta and mild clinical expression in the heterozygotes. Hum Genet 99:478–483
Fassihi H, Diba VC, Wessagowit V, Dopping-Hepenstal PJ, Jones CA, Burrows NP, McGrath JA (2005) Transient bullous dermolysis of the newborn in three generations. Br J Dermatol 153:1058–1063
Fine JD, Eady RA, Bauer EA, Briggaman RA, Bruckner-Tuderman L, Christiano A, Heagerty A, Hintner H, Jonkman MF, McGrath J, McGuire J, Moshell A, Shimizu H, Tadini G, Uitto J (2000) Revised classification system for inherited epidermolysis bullosa: report of the second international consensus meeting on diagnosis and classification of epidermolysis bullosa. J Am Acad Dermatol 42:1051–1066
Goto M, Sawamura D, Ito K, Abe M, Nishie W, Sakai K, Shibaki A, Akiyama M, Shimizu H (2006) Fibroblasts show more potential as a target cells than keratinocytes for COL7A1 gene therapy of dystrophic epidermolysis bullosa. J Invest Dermatol 126: (in press)
Hammami-Hauasli N, Schumann H, Raghunath M, Kilgus O, Luthi U, Luger T, Bruckner-Tuderman L (1998) Some, but not all, GS mutations in COL7A1 result in intracellular accumulation of collagen VII, loss of anchoring fibrils, and skin blistering. J Biol Chem 273:19228–19234
Jonkman MF, Moreno G, Rouan F, Oranje AP, Pulkkinen L, Uitto J (1999) Dominant dystrophic epidermolysis bullosa (Pasini) caused by a novel GS mutation in the type VII collagen gene (COL7A1). J Invest Dermatol 112:815–817
Mellerio JE, Salas-Alanis JC, Talamantes ML, Horn H, Tidman MJ, Ashton GH, Eady RA, McGrath JA (1998) A recurrent GS mutation, G2043R, in the type VII collagen gene (COL7A1) in dominant dystrophic epidermolysis bullosa. Br J Dermatol 139:730–737
Pulkkinen L, Uitto J (1999) Mutation analysis and molecular genetics of epidermolysis bullosa. Matrix Biol 18:29–42
Sawamura D, Yasukawa K, Kodama K, Yokota K, Sato-Matsumura KC, Tanaka T, Shimizu H (2002) The majority of keratinocytes incorporate intradermally injected plasmid DNA regardless of size but only a small proportion of cells can express the gene product. J Invest Dermatol 118:967–971
Sawamura D, Goto M, Yasukawa K, Sato-Matsumura K, Nakamura H, Ito K, Nakamura H, Tomita Y, Shimizu H (2005) Genetic studies of 20 Japanese families of dystrophic epidermolysis bullosa. J Hum Genet 50:543–546
Shimizu H, McGrath JA, Christiano AM, Nishikawa T, Uitto J (1996) Molecular basis of recessive dystrophic epidermolysis bullosa genotype/phenotype correlation in a case of moderate clinical severity. J Invest Dermatol 106:119–124
Shimizu H, Hammami-Hauasli N, Hatta N, Nishikawa T, Bruckner-Tuderman L (1999) Compound heterozygosity for silent and dominant glycine substitution mutations in COL7A1 leads to a marked transient intracytoplasmic retention of procollagen VII and a moderately severe dystrophic epidermolysis bullosa phenotype. J Invest Dermatol 113:419–421
Uitto J, Chung-Honet LC, Christiano AM (1992) Molecular biology and pathology of type VII collagen. Exp Dermatol 1:2–11
Wessagowit V, Ashton GH, Mohammedi R, Salas-Alanis JC, Denyer JE, Mellerio JE, Eady RA, McGrath JA (2001) Three cases of de novo dominant dystrophic epidermolysis bullosa associated with the mutation G2043R in COL7A1. Clin Exp Dermatol 26:97–99
Acknowledegments
The authors wish to thank Akari Nagasaki for her technical assistance, and also thank Dr James R. McMillan for proofreading and comments concerning this manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science and by a Health and Labor Sciences Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawamura, D., Sato-Matsumura, K., Shibata, S. et al. COL7A1 mutation G2037E causes epidermal retention of type VII collagen. J Hum Genet 51, 418–423 (2006). https://doi.org/10.1007/s10038-006-0378-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-006-0378-5