Abstract
Chromosomal translocations in T-cell malignancies frequently involve the T-cell receptor (TCR)α/δ locus at chromosome 14q11. Although 14q11 abnormalities are found in about 10% of adult T-cell leukemia (ATL) cases, until now there has been no direct evidence showing involvement of the TCR locus in ATL—a malignancy closely associated with HTLV-1 infection. The breakpoints of T-cell malignancies most commonly occur within the Jα or Jδ region of the TCR locus. In ATL, however, despite extensive searching no breakpoint has yet been found in that region. Using fluorescence in situ hybridization with a panel of cosmid and bacterial artificial chromosome probes derived from chromosome 14, including the variable region of the TCRα locus, comprehensive analysis of an ATL patient carrying inv(14)(q11q32) revealed that the TCR locus was indeed involved in this inversion. Molecular cloning of the breakpoint revealed the juxtaposition of TCR Vα to the 14q24 region as a result of two consecutive inversions: inv(14)(q11q32) and inv(14)(q11q24). We also found a gene near the breakpoint at the 14q24 region that is downregulated in this ATL patient and is assigned in the database as a pseudogene of ADAM21 (a disintegrin and metalloproteinase domain 21). Our expression analysis, however, showed that this pseudogene was actually expressed and was capable of encoding a protein similar to ADAM21; thus we have named this gene ADAM21-like (ADAM21-L).
Similar content being viewed by others
Introduction
Adult T-cell leukemia/lymphoma (ATL) is an aggressive lymphoid proliferation associated with HTLV-1, the first retrovirus associated directly with human malignancy (Poiesz et al. 1980; Hinuma et al. 1982). Because of its tight association with HTLV-1, most studies on leukemogenesis of ATL have focused on the function of HTLV-1. However, ATL develops only in a small proportion of HTLV-1-infected people (1 in 1,000) after a long latent period of 40–60 years (Tajima 1990; Yamaguchi et al. 2003). ATL cells are clonal with respect to viral integration, but approximately 30% of ATL cases exhibit deletions of the viral structural genes (Korber et al. 1991). More importantly, ATL cells do not express detectable levels of viral RNA or protein (Franchini et al. 1984; Yoshida et al. 1984), suggesting that the expression of HTLV-1 is needed only at the early stage of immortalization, and that changes in the expression of cellular genes are required to give rise to a tumor. Malignant transformation is thought to be associated with changes in the expression of several genes, and this alteration in gene expression is believed to be critical to the development of the malignant phenotype. Chromosome translocations are useful landmarks in the identification of such changes.
Cytogenetic abnormalities involving 14q11 are consistently observed in T-cell tumors. The frequency and phenotypic specificity of these cytogenetic aberrations suggest a causative role in leukemogenesis, presumably by interfering with the normal function of a gene near the site of chromosomal rearrangement. Involvement of the T-cell receptor (TCR)α/δ locus with a variety of chromosome abnormalities has been reported in many types of T-cell malignancies, and breakpoints in most of these abnormalities were found within the J or D region. Although 14q11 abnormalities have been found in 10% of ATL cases (Itoyama et al. 2001), and it has previously been postulated that the TCRα/δ locus might be involved in ATL (Sadamori et al. 1985; Isobe et al. 1990), no direct evidence in support of this hypothesis has been shown until now.
Here, we describe the first case report of TCR locus involvement in an ATL patient carrying inv(14)(q11q32). Cytogenetic analyses by fluorescence in situ hybridization (FISH) and molecular cloning of the breakpoint revealed that this rearrangement took place between a Vα segment and the 14q24 region, and actually resulted from two consecutive inversions: an inversion between 14q11 and 14q32, followed by another between the 14q11 and 14q24 region. We found a gene near the breakpoint with significant sequence similarity to the ADAM21 gene located at the 14q24 locus. Although assigned as a pseudogene in the database, we demonstrated this to be a true gene.
Materials and methods
Patient
A sample was obtained from the peripheral blood of an acute-type ATL patient at the time of diagnosis. Mononuclear cells separated by centrifugation over Ficoll–Hypaque gradients were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum without any mitogens. The percentage of lymphocytes in mononuclear cells was 30%. The frequency of leukemic cells in this ATL patient as determined by indented or lobulated nuclei in lymphocytes was 72%, and 27 out of 29 metaphases (93%) analyzed showed a basically identical karyotype of 45, X, -Y, add(1)(q10), t(1;3)(p12;q25), +i(1)(q10), add(7)(p22), −10, del(13)(q22q32), inv(14)(q11q32), add(21)(p11), −22, +mar [27]/46, XY [2]. The surface markers of fresh leukemic cells were CD2+/CD3+/CD4+/CD5+/CD8−/CD25+/ and CD19−.
DNA probes
In situ hybridization was performed using the following probes with their representative locations: BAC367C1 (Vα1.1–Vα1.2); cosmid clones 8cos4 (Vα2–Vα3), 8cos77 (Vα4–Vα6), and PLcos22 (Cα); probe PLC4 specific to the region distal to breakpoint cluster of TCL1 locus at 14q32.1; and D14S16 probe specific to the marker D14S16 at 14q32.2. The bacterial artificial chromosome (BAC) clone 367C1 was purchased from Research Genetics (Huntsville, AL). The cosmid clones 8cos4 and 8cos77, and probe D14S16 were isolated by screening cosmid libraries constructed with DNA from either CEPH Mega YAC844D7 or human placenta as described (Isobe et al. 1988) using Vα or D14S16 probe. Probes PLC4 and PLcos22 were previously described (Isobe et al. 1988; Narducci et al. 1995). For Southern hybridization, probes included: Vα2- and Vα3-specific probes corresponding to PCR products derived from genomic DNA with specific sets of primers (5′-AGGTGGCGGAGGTTACATAA-3′/5′-CCCGCACCTGGAGGATGAGC-3′) and (5′-AGGCCCAACGTGGAGAAAAT-3′/5′-GTCGCTCACAAGGGCAGATG-3′), respectively, and probe pM19 (Isobe et al. 1990) specific for the Jα segment.
DNA sequencing
DNA sequencing was performed on an ABI automated sequencer (ABI373; Applied Biosystems, Foster City, CA), and analyzed using Sequencher (Gene Codes, Ann Arbor, MI) and Genetyx software (GENETYX Corporation, Tokyo, Japan). BLAST searches were performed on the web-based application.
Fluorescence in situ hybridization
Metaphase slides were hybridized in situ with labeled probes. Probes were labeled with biotin or digoxigenin by nick translation as described (Narducci et al. 1995). Briefly, 300 ng labeled probe was used for each experiment, and hybridization was performed at 37°C in a total volume of 10 μl containing 2×SSC, 50% formamide, 10 mM Na3PO4, 10% dextran sulfate, 0.5 μg Cot1 DNA (Invitrogen, Carlsbad, CA) and 20 μg sonicated salmon sperm DNA and tRNA each. Post-hybridization washing was performed in 2×SSC containing 50% formamide at 37°C for 15 min followed by 2×SSC for 5 min at 37°C. Immunological detection of biotin- and digoxigenin-labeled DNA was performed with fluorescein isothiocyanate (FITC)-conjugated avidin (green signal) and rhodamin-labeled antidigoxigenin (red signal), respectively. Examinations for band localization of probe were carried out with a fluorescence microscope after counterstaining with DAPI (0.5 μg/μl) in 2×SSC.
Adaptor ligated-PCR
Adaptor ligated-PCR (AL-PCR) for the cloning of rearrangements was performed essentially as described previously (Ozawa et al. 2004). Genomic DNA (500 ng) from the ATL patient was partially digested with 0.1 U Sau3AI in a 75 μl reaction mixture containing 5 mM MgCl2, 50 mM KCl in 20 mM Tris–HCl (pH 7.4) for 1 h at 37°C, followed by a partial fill-in reaction using 33 μM each of dA, dG, and 0.5 μl Klenow enzyme (5 U/μl) in a 50 μl reaction mixture containing 10 mM MgCl2, 10 mM dithiothreitol (DTT), 50 mM Tris–HCl (pH 7.6) for 30 min at 37°C. A set of adaptors, 5′-CTAATACGACTCACTATAGGGCTCGAGCGGCCGCCCGGGCAGGT-3′ and 5′PO4-TCACCTGCCC-NH2-3′, 2 μM each, was ligated to the DNA fragment (100 ng) using 250 U T4 DNA ligase (Nippon Gene, Toyama, Japan) in a 5 μl ligation mixture containing 9.4% polyethylene glycol, 310 mM NaCl, 15 mM MgCl2, 30 mM dithiothreitol, 1.6 mM ATP in 80 mM Tris–HCl (pH 7.9). Incubation was carried out at 37°C for 2 h. Using one-fortieth of the ligated DNA as PCR template, nested amplification was carried out with AP1 and V16-α3-1-long-primer or Jα32_L1 for the first PCR and AP2 and V16-α3-2-long-primer or Jα32_L2 for the second PCR according to protocol supplied by the manufacturer. AP1: 5′-CCATCCTAATACGACTCACTATAGGGC-3′; AP2: 5′-ACTCACTATAGGGCTCGAGCGGC-3′; V16-α3-1-long-primer: 5′-TCTGCACCCATCTCGATGCTTGCGATGCTCTTCA-3′; V16-α3-2-long-primer: 5′-TCCCCTTCCCATTTTCCACTCGCATGATCCACTT-3′; Jα32_L1: 5′-AAGGGTGCTGTCGCTTCCTACTT-3′; Jα32_L2: 5′-TGGCTGGACAGCAAGCAGAGTG-3′.
RT-PCR
RT-PCR was performed using a number of MTC-cDNAs (Clontech) including spleen, testis, small intestine and leukocyte as templates according to the manufacturer’s protocol. Primers 5′-CAAGGGCAACAGGCATAAAC-3′ and 5′-GGTCCCATCCACTGCCATTA-3′ derived from 5′ UTR of the ADAM21-like gene were used for the amplification. The resulting PCR products were digested with HinfI and the gene expression level was analyzed by gel electrophoresis. Genomic contamination was checked with primers 5′-CGGGGAGGATCACATTTCCA-3′ and 5′-TGCCAGATTTGCTCCCTGTG-3′, both derived from 14q24 genomic sequence.
Southern blot
Genomic DNA from the ATL patient was extracted and subjected to BamHI digestion, gel electrophoresis, and Southern blot analysis as previously described (Southern 1975), using DNA from normal placenta as control. Hybridization was carried out using Vα and Jα probes corresponding to BamHI restriction fragments as indicated in Fig. 3a. Probes were labeled with 32P-dCTP using a Megaprime DNA labeling system (Amersham Biosciences, Piscataway, NJ) according to manufacturer’s specifications.
PCR confirmation of rearrangement
The rearrangement detected by Southern blot was confirmed by PCR using primers derived from Vα3 (Vα3_S: 5′-ATATCCCCTTCCCATTTTCCA-3′; Vα3_AS: 5′-AGCACAGAAGTACAAAGCGGAGTC-3′) and 14q24 (BAC486O13_11215U: 5′-CGGGGAGGATCACATTTCCA-3′; BAC486O13_12342L: 5′-TGCCAGATTTGCTCCCTGTG-3′) according to the manufacturer’s protocol.
Transfection of COS-7 cells
The mammalian COS-7 cell line was transfected with cDNA encoding the ADAM21-like protein using expression vector pEGFP-N1 (Clontech). Cell culture and analysis for green fluorescent protein (GFP) expression were performed as described previously (Saitou et al. 2000).
Results
Molecular cytogenetic analysis of inverted chromosome 14
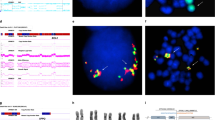
Cytogenetic analysis of the ATL patient revealed two different alleles of chromosome 14: an apparently normal chromosome and an inverted chromosome. The TCRα/δ locus was investigated by FISH using a pool of BAC and cosmid probes specific to the variable regions Vα1, Vα2–3, Vα4–6 and constant region Cα as indicated (Fig. 1). From the FISH analysis, both Cα and Vα1 were centromeric in the normal chromosome, while the hybridization signal from the Cα-specific probe PLcos22 was found to be telomeric in the inverted chromosome, thus confirming the inversion within the TCR locus (Fig. 2a). The Vα2–3 region was retained in the inverted chromosome, as observed with the hybridization signal from the 8cos4 probe (Fig. 2b). However, no signal was detected with the Vα4–6 probe in either allele, indicating deletion of the region downstream of Vα3 during VJ rearrangement (Fig. 2c). As the TCL1 locus was frequently found to be translocated with TCR genes in T-cell leukemias, we used the PLC4 probe to investigate whether TCL1 was involved in the rearrangement. In the inverted allele, hybridization revealed the telomeric breakpoint to be distal to the TCL1 locus. In the event of simple inversion, the PLC4 signal should be centromeric; interestingly, the signal from PLC4 was found to be one-third telomeric, which strongly suggested that a complex secondary inversion took place (Fig. 2d). Moreover, hybridization with the D14S16 probe specific to 14q32.2 showed this locus to be distal to the telomeric breakpoint (Fig. 2e). Thus the telomeric breakpoint was mapped telomeric to TCL1 and centromeric to the D14S16 locus. Although the origin of the marker chromosome found in this patient was not determined by our cytogenetic analysis, FISH analysis revealed this marker chromosome to be actually derived from inverted chromosome 14. In this marker chromosome, however, with the exception of the Cα and D14S16 probes, no signal was detected from any other probes suggesting deletion of the respective regions.
Representative FISH (pseudocolored) and G-band (DAPI stained) images obtained upon analysis of metaphase spreads from adult T-cell leukemia (ATL). TCRα/δ (14q11)-specific probes 367C1, 8cos4, 8cos77 and PLcos22 correspond to Vα1, Vα2–3, Vα4–6, and Cα, respectively, while probes PLC4 and DI4S16 represent the region distal to breakpoint cluster of TCL1 (14q32.1) and the D14S16 marker (14q32.2), respectively. a Both 367C1(green) and PLcos22 (red) probes were centromeric in normal chromosome; in the allele with the inversion, the hybridization signal from PLcos22 was telomeric. b Signal from 8cos4 (green) was observed in both normal and inverted chromosome. c PLcos22 (red) signal was found in both chromosomes while there was no signal from 8cos77 (green). d Signal from PLC4 (green) was telomeric instead of being centromeric in the inverted chromosome. e D14S16 (red) signal was distal to the telomeric breakpoint. No signal was detected from any probes other than PLcos22 and D14S14 in the marker chromosome
Identification of rearrangements by Southern blot analysis
Based on the FISH results, Southern hybridization to detect and map the translocation breakpoint was performed with probes derived from Vα2, Vα3 and Jα regions as shown in Fig. 3a. Consistent with the FISH results, hybridization with the Vα2 probe showed no rearrangement, generating a germline 14.2 kb BamHI fragment in both normal and ATL, whereas the Vα3 probe revealed a single rearranged band of 5.2 kb in ATL in addition to the 7.5 kb germline band (Fig. 3b). With the Jα-specific probe pM19, two germline BamHI bands of 6 and 3.6 kb were found in both cases, with an additional 14.2 kb band in ATL.
Detection of rearrangements by Southern blot analysis. a BamHI restriction map of specific Vα and Jα regions, showing the respective size and location of the probes used (different scales are shown). b Southern hybridization of BamHI-digested genomic DNA from normal placenta and from an ATL patient with probes as indicated. Germline and rearranged bands are indicated with dark and light arrowheads, respectively
Molecular cloning of breakpoint and analysis of locus containing translocation
We performed cloning by AL-PCR to characterize the Vα3 and Jα rearrangements detected by Southern hybridization. Sequence analysis of the PCR product extending from Jα32 revealed that the pM19-detected rearrangement resulted from VJ recombination between Vα2 and Jα32 with the addition of nine nucleotides (Fig. 4a). Although no rearrangement was detected by Southern analysis with the Vα2 probe, comparison of the restriction map with the resulting rearrangement revealed that the rearranged 14.2 kb band detected by pM19 comigrated with the 14.2 kb germline band detected with the Vα2 probe. On the other hand, sequence analysis of the PCR product corresponding to the rearrangement detected by Vα3 revealed this rearrangement to have resulted from recombination between Vα3 and an unknown region; a GenBank data search revealed this sequence to be derived from 14q24. The latter rearrangement caused chromosomal translocation without involving the recombination signal sequence (RSS) near the Vα3 locus, with insertion of a single C nucleotide at the breakpoint (Fig. 4b). To confirm the rearrangement, we performed PCR with primers derived from Vα3 and 14q24 region (Fig. 5a); both wild type and ATL generated bands of 349 and 1,147 bp (Fig. 5b, lanes A and B). The 692 bp band was found only in ATL with a combination of primers (Vα3_S and BAC486013_11215U), which confirmed the reciprocal rearrangement involving Vα3 and 14q24. However, no amplification was found with the other combination (Vα3_AS and BAC486013_12342L), suggesting deletion of the 3′ region of Vα3 as a result of the rearrangement.
Schematic representation of rearrangements from normal VJ joining (a) and inv(14) in the ATL case (b). The germline Vα and Jα sequences with corresponding recombination signal sequence (RSS) are shown; the resulting addition of nucleotides from the rearrangements is marked with (+). Breakpoints in the ATL chromosome are indicated with arrows
A gene is located near the translocation breakpoint
From the database, we found several ESTs near the breakpoint, namely AA406146, AA776743, AW119192 and AI125380. We also found the pseudogene of ADAM21, about 4 kb centromeric to the breakpoint, overlapping the intronic sequences of the first three ESTs (Fig. 6); ADAM21 is located about 200 kb telomeric to the breakpoint. Primers derived from these ESTs did not reveal any expression in normal tissues, including leukocytes, or in ATL samples, which led us to focus on the pseudogene near the breakpoint. We performed RT-PCR with primers derived from the 5′ UTR of the ADAM21 pseudogene (Fig. 7a) to examine its expression in comparison with ADAM21. The lower bands (202 and 218 bp) represented ADAM21 while the higher bands (249 and 252 bp) originate from its pseudogene—each band corresponding to a Hinf1 digested fragment. As shown in Fig. 7b, we found expression of both genes in all tissues examined; the level of ADAM21 pseudogene expression was slightly less than that of ADAM21 in general. In contrast, expression of both genes was similar in resting T-cells and other ATL-derived cell lines, namely RST1, ST1 and ATN1. Activated T-cells also showed similar expression (data not shown), while our ATL case showed significantly reduced expression of pseudogene.
RT PCR analysis and structural comparison of ADAM21 and ADAM21-L. a Primers derived from the 5′ UTR were used for expression analysis; HinfI restriction sites are indicated within each gene. b PCR products were run on acrylamide gel after digesting with HinfI. Placenta positive control, genomic DNA from human placenta; dH2O negative control (top). Genomic contamination was checked with primers derived from 14q24 (bottom). c Comparison of ADAM21 and ADAM21-L proteins with respect to domain structures. d Transfection of COS7 cell showing nuclear and cytoplasmic localization of ADAM21-L
Owing to its significant similarities in structure and expression profile with ADAM21, we call this pseudogene ADAM21-like (ADAM21-L). Figure 7c gives a comparison of motifs in the two genes; the ADAM21-L protein shows considerable sequence homology with ADAM21, both having a single exon in their ORF. When transfected into COS7 cell, ADAM21-L was found to be localized within the cytoplasm and nucleus (Fig. 7d).
Discussion
Nonrandom chromosomal translocations are characteristic of most human hematopoietic malignancies (Haluska et al. 1989). In B and T-cells, chromosomal translocations and inversions often occur as a consequence of mistakes during the normal process of recombination of the genes for immunoglobulins or TCRs. These rearrangements juxtapose enhancer elements of the immunoglobulin or TCR genes to oncogenes, expression of which is then deregulated (Croce 1987; Isobe et al. 1988); in some cases, such a translocation may also cause disruption of a tumor suppressor gene (Duro et al. 1996).
We have shown evidence of a TCR gene rearrangement in an ATL patient. As summarized in Fig. 8, with the initial inversion, the region between TCR Vα locus and 14q32 is flipped upside down, with Cα positioned centromeric and proximal to the D14S16 locus at 14q32.2, while a region further telomeric to the TCL1 locus at 14q32.1 is juxtaposed to a region downstream of Vα3. The latter rearrangement involves Vα3 and the 14q24 region centromeric to ADAM21-L. Following duplication, a large segment between a region centromeric to Vα1 and near-center of the long arm of chromosome 14 was found to be deleted in one of the inverted chromosomes identified as the marker chromosome in cytogenetic analysis.
Schematic model showing the consecutive inversions occurring within chromosome 14 in ATL. Chromosomal regions involved in the rearrangement are indicated with boxes, with arrows indicating the orientation of the respective regions. The initial rearrangement inv(14)(q11q32) involved a region Cα upstream of the TCR locus and the region telomeric to TCL1 at 14q32.1. The second inversion occurred between Vα3 and 14q24. The marker chromosome with the region deleted after duplication is also shown
We cloned the breakpoint of inv(14)(q11q24) from our patient and found that the Vα gene was indeed involved. While Vα involvement has been reported in very few cases, such as in secondary leukemia (Bernard et al. 1988), ATL is often characterized by atypical rearrangements involving the Vα gene. A complex rearrangement with inversion within the TCRα locus was previously found in ATL, where the rearrangement resulted as a consequence of hybrid joint formation, a type of faulty rearrangement between the RSS and the coding sequence (Saitou et al. 2001). Sequence analysis in our patient showed the absence of conserved heptamer and nonamer signal sequences at the breakpoint junction, which implicated factors other than faulty recombination. Moreover, since ATL cells are mature T-cell malignancies with a phenotype of CD3+, CD4+/CD8−, the TCRα and β rearrangements have already been completed on both alleles at the time of leukemogenesis. Therefore, it is less likely that the faulty VJ joining of the TCRα locus during T-cell differentiation causes translocation in ATL (Saitou et al. 2001). However, the Vα region is still accessible for rearrangements in mature T-cells (Marolleau et al. 1988), thus rendering it a major target for translocation in ATL. Previous failures to identify TCR locus involvement in translocation were largely attributed to the large and complex Vα region, which spans 1 Mb, as well as the absence of VJ joining in the mature ATL cell. Our ATL case was extensively analyzed and an HTLV-1 integration site was found in 9q12, but no integration site was found within the TCR gene locus (Ozawa et al. 2004). Thus integration of HTLV1 is not related to this inversion event.
In T-prolymphocytic leukemia (T-PLL), TCR loci are often translocated with regions centromeric or telomeric to the TCL1 locus at 14q32.1, leading to deregulation of the TCL1 gene (Narducci et al. 1995). In our ATL patient, however, precise FISH analysis showed the breakpoint to be located at least several megabases telomeric to the TCL1 locus (data not shown). Thus, TCL1 was less likely to be involved in this case. Further characterization of the breakpoint of the initial inversion inv(14)(q11q32) may help identify the gene associated with ATL leukemogenesis. We have described the gene near the breakpoint: ADAM21-L is similar to ADAM21—a member of the ADAM protein family encoding a disintegrin and metalloprotease (ADAM) domain 21. Members of this family are membrane-anchored proteins structurally related to snake venom disintegrins that have been implicated in a variety of biologic processes involving cell–cell and cell–matrix interactions, including fertilization, muscle development, and neurogenesis. ADAM21 gene expression was previously found by northern analysis to be testis specific (Hooft van Huijsduijnen 1998). However, using RT-PCR—a more sensitive method of detecting transcripts—both the ADAM21 and ADAM21-L genes showed low but almost ubiquitous expression. We identified an ORF within the ADAM21-L gene sequence, and analyzed the ADAM21-L cDNA using an expression vector fused with GFP. Transfection of this expression construct into COS7 cells resulted in fluorescence indicating successful initiation of translation from the ADAM21-L gene transcript. We used the web-based program SOSUI (http://www.sosui.proteome.bio.tuat.ac.jp/sosuiframe0.html) to analyze the predicted cellular localization of the ADAM21-L and ADAM21 gene products. ADAM21-L was found to possess 2 transmembrane domains with no signal sequence at the N-terminal end, compared to 3 transmembrane domains with a signal sequence in ADAM21. This difference in the N-terminal structure explains the observation that ADAM21-L is observed in the nucleus as well as the cytoplasm, while the ADAM21 protein is localized to the cellular membrane (Yang et al. 2005).
We studied expression of the ADAM21-L gene using ATL-derived cell lines (Fig. 7b). Unlike our ATL case, however, we found comparable expression of ADAM21-L and ADAM21 in these samples. Thus it is difficult to draw a conclusion as to whether this gene is universally deregulated in ATL. Nevertheless, as in our ATL patient, downregulation of ADAM21-L may be found in isolated cases of ATL. Additional studies are warranted to establish the role of this gene in ATL leukemogenesis.
References
Bernard O, Larsen CJ, Hampe A, Mauchauffe M, Berger R, Mathieu-Mahul D (1988) Molecular mechanisms of a t(8;14)(q24;q11) translocation juxtaposing c-myc and TCR- alpha genes in a T-cell leukemia: involvement of a V alpha internal heptamer. Oncogene 2:195–220
Croce CM (1987) Role of chromosome translocations in human neoplasia. Cell 49:155–156
Duro D, Bernard O, Della Valle V, Leblanc T, Berger R, Larsen CJ (1996) Inactivation of the P16INK4/MTS1 gene by a chromosome translocation t(9;14)(p21–22;q11) in an acute lymphoblastic leukemia of B-cell type. Cancer Res 56:848–854
Franchini G, Wong-Staal F, Gallo RC (1984) Molecular studies of human T-cell leukemia virus and adult T-cell leukemia. J Invest Dermatol 83:63s–66s
Haluska FG, Tsujimoto Y, Russo G, Isobe M, Croce CM (1989) Molecular genetics of lymphoid tumorigenesis. Prog Nucleic Acid Res Mol Biol 36:269–280
Hinuma Y, Komoda H, Chosa T, Kondo T, Kohakura M, Takenaka T, Kikuchi M, Ichimaru M, Yunoki K, Sato I, Matsuo R, Takiuchi Y, Uchino H, Hanaoka M (1982) Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer 29:631–635
Hooft van Huijsduijnen R (1998) ADAM 20 and 21; two novel human testis-specific membrane metalloproteases with similarity to fertilin-alpha. Gene 206:273–282
Isobe M, Sadamori N, Russo G, Shimizu S, Yamamori S, Itoyama T, Yamada Y, Ikeda S, Ichimaru M, Kagan J et al (1990) Rearrangements in the human T-cell-receptor alpha-chain locus in patients with adult T-cell leukemia carrying translocations involving chromosome 14q11. Cancer Res 50:6171–6175
Isobe M, Russo G, Haluska FG, Croce CM (1988) Cloning of the gene encoding the delta subunit of the human T-cell receptor reveals its physical organization within the alpha-subunit locus and its involvement in chromosome translocations in T-cell malignancy. Proc Natl Acad Sci USA 85:3933–3937
Itoyama T, Chaganti RS, Yamada Y, Tsukasaki K, Atogami S, Nakamura H, Tomonaga M, Ohshima K, Kikuchi M, Sadamori N (2001) Cytogenetic analysis and clinical significance in adult T-cell leukemia/lymphoma: a study of 50 cases from the human T-cell leukemia virus type-1 endemic area, Nagasaki. Blood 97:3612–3620
Korber B, Okayama A, Donnelly R, Tachibana N, Essex M (1991) Polymerase chain reaction analysis of defective human T-cell leukemia virus type I proviral genomes in leukemic cells of patients with adult T-cell leukemia. J Virol 65:5471–5476
Marolleau JP, Henni T, Gaulard P, Le Couedic JP, Gourdin MF, Divine M, Katz A, Tulliez M, Goossens M, Reyes F et al (1988) Hairy cell leukemia associated with large granular lymphocyte leukemia: immunologic and genomic study, effect of interferon treatment. Blood 72:655–660
Narducci MG, Virgilio L, Isobe M, Stoppacciaro A, Elli R, Fiorilli M, Carbonari M, Antonelli A, Chessa L, Croce CM, Russo G (1995) TCL1 oncogene activation in preleukemic T-cells from a case of ataxia-telangiectasia. Blood 86:2358–2364
Ozawa T, Itoyama T, Sadamori N, Yamada Y, Hata T, Tomonaga M, Isobe M (2004) Rapid isolation of viral integration site reveals frequent integration of HTLV-1 into expressed loci. J Hum Genet 49:154–165
Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC (1980) Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA 77:7415–7419
Sadamori N, Kusano M, Nishino K, Tagawa M, Yao E, Yamada Y, Amagasaki T, Kinoshita K, Ichimaru M (1985) Abnormalities of chromosome 14 at band 14q11 in Japanese patients with adult T-cell leukemia. Cancer Genet Cytogenet 17:279–282
Saitou M, Sugimoto J, Hatakeyama T, Russo G, Isobe M (2000) Identification of the TCL6 genes within the breakpoint cluster region on chromosome 14q32 in T-cell leukemia. Oncogene 19:2796–2802
Saitou M, Sadamori N, Isobe M (2001) Identification of an aberrant type of rearrangement in the T-cell receptor alpha/delta locus in adult T-cell leukemia. J Hum Genet 46:706–711
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Tajima K (1990) The 4th nation-wide study of adult T-cell leukemia/lymphoma (ATL) in Japan: estimates of risk of ATL and its geographical and clinical features. The T- and B-cell Malignancy Study Group. Int J Cancer 45:237–243
Yamaguchi K, Yonemura Y, Okabe H, Takahama Y, Nagai S, Yamaguchi H, Hirai K (2003) Detection of anti-human T-lymphotropic virus type I antibody in whole blood by a novel counting immunoassay. Clin Chem 49:275–280
Yang P, Baker KA, Hagg T (2005) A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. J Comp Neurol 490:163–179
Yoshida M, Seiki M, Yamaguchi K, Takatsuki K (1984) Monoclonal integration of human T-cell leukemia provirus in all primary tumors of adult T-cell leukemia suggests causative role of human T-cell leukemia virus in the disease. Proc Natl Acad Sci USA 81:2534–2537
Acknowledgements
This research was partially supported by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan. We would like to thank Dr. Masumichi Saitou for providing us with the cosmid clones specific to Vα locus.
Author information
Authors and Affiliations
Corresponding author
Additional information
The ADAM21-L gene and breakpoint sequences have been deposited with the GenBank database under accession numbers DQ202398 and DQ302756, respectively.
Rights and permissions
About this article
Cite this article
Haider, S., Hayakawa, K., Itoyama, T. et al. TCR variable gene involvement in chromosome inversion between 14q11 and 14q24 in adult T-cell leukemia. J Hum Genet 51, 326–334 (2006). https://doi.org/10.1007/s10038-006-0364-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-006-0364-y