Abstract
To investigate the involvement of uniparental disomies (UPDs) in spontaneous abortion, the polymorphic patterns of microsatellites on each chromosome were analyzed in 164 cases of abortion. Eighty-three of the 164 cases had chromosomal abnormalities. In 79 of the remaining 81 cases with normal karyotypes, the microsatellite analysis revealed that biparental patterns were present in the informative microsatellites in all chromosomes. In one of the remaining two cases, however, the polymorphic patterns of chromosome 14 appeared to be both of paternal origin. The patterns of the distal of the long arm were homozygous, and those of the remaining region were heterozygous. That is, this fetus had paternal UPD 14, originating from meiosis I nondisjunction. In the other case, the polymorphic patterns of the distal one third of the long arm of chromosome 7 were uniparental (maternal) in origin whereas those of the remaining region of this chromosome were biparental. These findings thus suggested that this chromosome might have originated from chromatid exchange between the long arms of paternal and maternal chromosome 7 at the first mitotic division. Microsatellite analysis, however, produced no evidence of duplication or deletion of any segments. The findings also suggest the possibility that some UPDs may cause spontaneous abortion.
Similar content being viewed by others
Introduction
A high percentage (around 15%) of recognized pregnancies end in spontaneous abortion. About half of these abortions have various kinds of chromosomal abnormalities such as aneuploids, polyploids, and monosomy of the X-chromosome (Hassold et al. 1980; Kajii et al. 1980; Warburton et al. 1980). The cause of the remaining cases of abortions of fetuses with a normal karyotype is mostly unknown, but immunological and other defects have been detected in some cases (Gill 1986; Kaider et al. 1999). However, the finding that typical chromosomal abnormalities account for a large portion of the causes of spontaneous abortions leads to the possibility that functional, structural, and constitutional abnormalities that are undetectable by the usual chromosomal analysis may also contribute to these abortions with a normal karyotype. These include, for example, cases with a deletion of fine chromosomal segments including a gene essential to fetal development, abnormal inactivation of the X-chromosome, or uniparental disomy (UPD) of chromosomes having an imprinting region.
Chromosomal abnormality in UPD cases cannot usually be detected by banding, except for a few cases with the phenotypically polymorphic chromosomes. To date, few UPD cases have been found among spontaneous abortions (Fritz et al. 2001; Kondo et al. 2004). To investigate the involvement of UPDs, we analyzed the chromosomal origin of spontaneous abortions in detail using microsatellite polymorphic markers and found UPD of chromosome 14 and a unique exchange of chromosome 7 in cases of spontaneous abortion.
Materials and methods
Cases of spontaneous abortion
Of the 164 cases of spontaneous abortion analyzed, 133 patients that aborted a fetus were admitted to the Department of Obstetrics and Gynecology, Nagoya City University School of Medicine, Nagoya, Japan. The remaining 31 cases were obtained from the Cell Bank constructed with a Health Science Research Grant for Research on Human Genome (H10-Genome-008) from the Ministry of Health, Labor and Welfare of Japan. These cases were aborted at 6–9 weeks of gestation. All of the patients and their spouses in these cases agreed to allow the use of parental and fetal materials for analysis, after being given understandable and detailed information on this study and its purposes. Peripheral blood of the patient with the spontaneous abortion and her spouse, and chorionic villi from the abortion, were obtained for each case. This study was approved by the IRB of the Institute for Developmental Research, Aichi Human Service Center, and the IRB of Nagoya City University Medical School.
Chromosomal analysis
The tissue of chorionic villi was separated under a stereomicroscope from three different parts of the samples from each abortion and was cultured separately using AmnioMAX C-100 medium (Invitrogen). All specimens were cultured within 18 h following abortion sampling. Cells were harvested for the chromosomal preparation at 6–19 days of cultivation. Peripheral blood lymphocytes from the patient with the abortion and from her spouse were also cultured and harvested conventionally for chromosomal analysis. Chromosomes were analyzed by G and Q banding.
Polymorphic analysis of microsatellites
Genomic DNA was extracted from the chorionic villi of the abortions and the blood of the patients with abortion and their spouses by the standard method. Two hundred polymorphic microsatellite markers on about every 20 Mb in all autosomes and the X-chromosome were selected from the Genethon collections (Dib et al. 1996). Some of the primers used in this study were provided by Prof. Y. Nakamura, Institute of Medical Sciences, University of Tokyo, Tokyo, Japan, and the others were synthesized. The microsatellite polymorphic patterns of the fetus and the parents for each marker locus were determined using a DNA-sequencer-assisted method with fluorescent microsatellite marker DNAs (Mansfield et al. 1994) with slight modifications (Fujimoto et al. 1998). When a polymorphic pattern suggesting disomy was obtained, further analysis using other synthesized primers that can detect many microsatellites in the same chromosome region was performed to clearly identify the parental origin of the chromosome.
Results
Of the 164 cases of spontaneous abortions investigated, 83 had chromosomal abnormalities, which were various but similar to those seen frequently in spontaneous abortions. In the remaining 81 cases with a normal karyotype—46,XX in 40 cases and 46,XY in 41 cases—polymorphic analysis of microsatellites was performed. The polymorphic analysis of villi from 79 of the 81 cases revealed that the informative microsatellite patterns of all of the autosomes, and of the X-chromosome of the 46,XX cases, were biparental patterns: one paternal (pat) and the other maternal (mat). These results indicate that every paired chromosome originated from one pat chromosome and one mat chromosome.
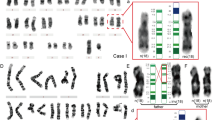
In one (case 35) of the remaining two 46,XX cases, the microsatellite polymorphic patterns of all paired autosomes except chromosome 14, and the X-chromosome were one pat and one mat each; however, the informative patterns of the three microsatellites of chromosome 14 were dual pat. Further polymorphic analysis of this case was performed using other synthesized primer sets for chromosome 14. Thirteen microsatellites showed informative patterns, clearly indicating both pat origin (Fig. 1), and all patterns of the other 17 microsatellites were consistent with pat origin. The results also showed that the microsatellite patterns in the region from the centromere to about two thirds of the chromosomal length away from the centromere of chromosome 14 (D14S261–D14S983) were heterozygous while those in the remaining region from that point to the distal end (D14S1058–D14S1010) were homozygous. That is, the results indicated that this fetus had pat iso- and heterodisomy of chromosome 14. The constitution of chromosome 14 in this case also suggests that both chromosomes 14 originated from pat meiosis I nondisjunction of dyad 14 that accompanied a crossover at a point about two thirds of the long arm away from the centromere.
Polymorphic patterns of microsatellites of chromosome 14 seen in the aborted fetus and the parents in case 35. a List of primers that showed informative patterns of microsatellite polymorphism, and the size of PCR products (bp). The arrangement of markers is roughly shown. b Polymorphic patterns of microsatellites in the fetus (case 35) and the parents. The polymorphic analysis indicates that this is a case of paternal iso-/heterodisomy of chromosome 14
In the other 46,XX case (case 107), on the other hand, polymorphic analysis of microsatellites revealed that the distal one third of the long arm of chromosome 7 appeared to be mat uniparental in origin whereas the segments from the distal part of the short arm to about two thirds distal from the centromere of the long arm of chromosome 7 were biparental in origin (Fig. 2). The microsatellite polymorphic patterns of the X-chromosome and all autosomes except for chromosome 7 were one pat and one mat. The segments of the distal part of the long arm of chromosome 7 that appeared to be both mat showed isodisomy. This would seem to suggest that one of the chromosomes 7 might have originated from an exchange between chromatids of the long arms of pat and mat chromosome 7 at the first mitotic division. Polymorphic analysis of microsatellites also revealed no evidence of the presence of cells with other chromosome constitutions derived from such chromatid exchange at the first mitotic division. Detailed investigation of the area around the breakpoint of the exchange revealed that, although all of the informative patterns obtained by analysis using primers from D7S2519 to those existing in more centromeric regions of the long arm of chromosome 7 were biparental, all of the informative patterns in all primers from D7S512 to those in the distal side of the long arm showed isodisomy. Further analysis using other primers between D7S2519 and D7S512 revealed no informative patterns. In addition, as a result of microsatellite analysis, there was no evidence of duplication or deletion of any segments around the breakpoint of the exchange.
Polymorphic patterns of microsatellites of chromosome 7 seen in the aborted fetus and the parents in case 107. a List of primers that showed informative patterns, and the size of PCR products (bp). The arrangement of markers and the locus of the centromere are roughly shown. b Polymorphic patterns of microsatellites in the fetus (case 107) and the parents. The polymorphic analysis indicates that this is a case of partial mat isodisomy of chromosome 7. There was no evidence of deletion or duplication of any segments around the breakpoint of exchange
Case 35 was the first pregnancy of a 32-year-old woman. Her spouse was 34 years old. The fetus was diagnosed as having a stopped heartbeat in the eighth week of pregnancy and aborted during the following week (8w5d) in a typical spontaneous abortion in which the fetus could not be found. There was nothing remarkable either before or during the pregnancy. The karyotypes of the woman and her spouse were normal. Case 107 was from the first pregnancy of a 36-year-old woman. Her husband was 37 years old. The fetal heartbeat stopped at the seventh week of pregnancy, and the fetus was aborted the following week (7w5d) in a typical spontaneous abortion in which the fetus could not be found. There was nothing remarkable either before or during the pregnancy. The chromosomes of the woman and her husband were normal.
Discussion
Mat and pat UPDs for various chromosomes in humans have been identified in individuals by ascertaining medical problems. Findings of imprinting disturbances, non-Mendelian inheritance of recessive genes, and chromosomally abnormal patterns indicated UPD (Ledbetter and Engel 1995; Engel 1998). Among these, abnormal clinical features have been distinctly shown in both pat and mat UPDs of chromosomes 14 and 15 (Nicholls et al. 1989; Bottani et al. 1994; Cotter et al. 1997; Sanlaville et al. 2000). Abnormal clinical features have also been shown in UPDs of chromosomes 2, 7, and 16 of only mat origin (Kalousek et al. 1993; Kotzot et al. 1995; Johnston et al. 1996) and in UPDs of chromosomes 6 and 11 of only pat origin (Henry et al. 1991; Temple et al. 1995). In particular, serious clinical features have been described for some UPDs, such as mat UPD 2 associated with severe growth retardation, pulmonary dysplasia, and renal failure (Webb et al. 1996; Shaffer et al. 1997); and pat UPD 14, which shows the phenotypes of thoracic narrowing and skeletal dysplasia (Cotter et al. 1997; Kurosawa et al. 2002). In contrast, some such as UPD 1, 13, 21, and 22 have almost no clinical features with either pat or mat UPDs (Ledbetter and Engel 1995; Engel 1998; Morison and Reeve 1998). On the other hand, UPDs such as chromosomes 3, 12, or 17–19 have not been found in any case to date. These facts suggest the possibility that some UPD cases may exhibit abnormalities before birth. Whereas mouse studies have clearly indicated that some UPDs affect the development of embryos and the placenta (Ferguson-Smith et al. 1991), it has not been ascertained in humans whether UPDs affect embryogenesis and fetal development.
In the present study, we found the first case of pat UPD 14 in human spontaneous abortion. Human chromosome 14q shares synthetic homology with the distal half of mouse chromosome 12, in which there is the imprinting region. Georgiades et al. (2000) demonstrated that mice with UPD 12 resulted in parent-origin-specific developmental defects, and the placentomegaly and abnormality of maternal artery supply were likely to contribute to the progressive loss of pat UPD 12 fetus after E15.5. To determine the effects of human UPD 14, further data such as ultrasonographic findings of the placenta and fetus are important, but we failed to obtain detailed findings in the present study (case 35).
To the best of our knowledge, of the UPD 14 cases in liveborns reported to date in the literature, 37 were mat (Antonarakis et al. 1993; Papenhausen et al. 1995; Barton et al. 1996; Tomkins et al. 1996; Splitt and Goodship 1997; Harrison et al. 1998; Miyoshi et al. 1998; Hordijk et al. 1999; Martin et al. 1999; Ralph et al. 1999; Ginsburg et al. 2000; Manzoni et al. 2000; Sanlaville et al. 2000; Eggermann et al. 2001; Katahira et al. 2002; Cox et al. 2004) and 8 pat (Wang et al. 1991; Papenhausen et al. 1995; Walter et al. 1996; Cotter et al. 1997; McGowan et al. 2002; Coveler et al. 2002; Kurosawa et al. 2002; Offiah et al. 2003). As mentioned above, most cases of pat UPD 14 have characteristic and often serious clinical features, including blepharophimosis, small thorax, and joint contractures, while the main features of mat UPD 14 are low birth weight, poor postnatal growth, fleshy nasal tip, and scoliosis. The number of reports of UPD 14 suggests that the frequency of pat UPD 14 cases in liveborns is actually fewer than that of mat UPD cases. The difference in the frequencies between pat and mat UPD 14 cases might have resulted from a difference in the actual rate of occurrence or in the rate of selective elimination during embryogenesis and fetal development. On the assumption that UPDs are formed by fertilization between gametes nullisomic and disomic for the same chromosome, the frequency of pat and mat UPDs for the same chromosome might be equal. If, in cases of selective elimination, developmental defects of UPD fetuses appear also to be abortion, pat UPD 14 cases may be seen more frequently in spontaneous abortions than mat UPD 14 cases. Generally, chromosomal abnormalities actually seen in liveborns, such as trisomies 18 and 21, are also seen in spontaneous abortions at several times the rate in liveborns (Carr and Gedeon 1977; Hook and Hamerton 1977). These facts suggest that UPDs with congenital abnormalities seen in liveborns may become a cause of spontaneous abortions in the same way as trisomies do.
Until now, however, the relationship of UPDs to abortion has not been well understood. In the literature to date, only three cases of UPDs 9, 16, and 21 have been found among spontaneous abortions (Fritz et al. 2001; Kondo et al. 2004), and the present case is the first report of UPD 14 in a spontaneous abortion. In other reports, the association of UPDs with spontaneous abortions has not been found (Shaffer et al. 1998; Smith et al. 1998). The combined frequency of UPD cases from these four studies and the present study is 1.69% (4/236), which indicates a low incidence of UPD in spontaneous abortion. However, there is a methodological limitation to the polymorphic analysis of microsatellites. Even using this kind of analysis, for instance, the mosaic cases of UPDs due to the trisomy rescue mechanism could not be detected in the ascertainment of UPD cases in spontaneous abortions.
Another case found in the present study (case 107) is a very rare one that was produced by an exchange between the chromatids of the long arm of pat and mat chromosome 7. This case had partial mat UPD of 7q. It is well known that abnormal clinical features are shown in mat UPD 7, including this region (Kotzot et al. 2000; Hannula et al. 2001). Other than this case, there is no reported case of spontaneous abortion in which there was demonstrated to be an exchange between pat and mat chromosome. In liveborns, on the other hand, many cases with an exchange between pat and mat chromosomes have been reported. For example, an exchange between pat and mat chromosomes is one of the mechanisms in contiguous gene syndromes, such as Prader–Willi and Angelman syndromes (Robinson et al. 1998; Nicholls and Knepper 2001), in which partial UPD and deletion of segments resulted from unequal exchange between chromatids of pat and mat homologous chromosomes. Although in the present case we could not find any deletion or duplication near the breakpoint of the exchange of chromosome 7 by polymorphic analysis of microsatellites, there remains a possibility that further detailed analyses may in fact show deletion and duplication resulting from the exchange. Generally, an exchange between chromatids of two homologous chromosomes produces two kinds of cells, each with different chromosome constitutions. Whereas this case had only one of the two kinds of karyotypes expected, it is possible that the cell with the other karyotype could not increase because of disadvantage due to microdeletions, duplication, or UPD resulting from an exchange.
A very interesting question is how many abnormalities undetectable by the usual banding method, such as UPDs and somatic exchanges that were detected in the present study, are concerned with miscarriage. Although polymorphic analysis of DNAs including microsatellite polymorphic analysis is one effective means for such elucidation, the data based on these methods remain insufficient. To clarify the relationship between constitutional abnormalities including UPDs and spontaneous abortion, or the effects during the developmental stages in humans, further investigations of abortions using DNA polymorphic markers and other means are needed.
References
Antonarakis SE, Blouin JL, Maher J, Avramopoulos D, Thomas G, Talbot CC Jr (1993) Maternal uniparental disomy for human chromosome 14, due to loss of a chromosome 14 from somatic cells with t(13;14) trisomy 14. Am J Hum Genet 52:1145–1152
Barton DE, McQuaid S, Stalling R, Griffin E, Geraghty M (1996) Further evidence for an emerging maternal uniparental disomy for chromosome 14. Analysis of a phenotypically abnormal de novo Robertsonian translocation t(13;14) carrier. Am J Hum Genet (Suppl) 59:698
Bottani A, Robinson WP, DeLozier Blanchet CD, Engel E, Morris MA, Schmitt B, Thun-Hohenstein L, Schinzel A (1994) Angelman syndrome due to paternal uniparental disomy of chromosome 15: a milder phenotype? Am J Med Genet 51:35–40
Carr DH, Gedeon M (1977) Population cytogenetics of human abortuses. In: Hook EB, Porter IH (eds) Population cytogenetics: studies in humans. Academic Press, New York San Francisco London, pp 1–9
Cotter PD, Kaffe S, McCurdy LD, Jhaveri M, Willner JP, Hirschhorn K (1997) Paternal uniparental disomy for chromosome 14: a case report and review. Am J Med Genet 70:74–79
Coveler KJ, Yang SP, Sutton R, Milstein JM, Wu YQ, Bois KD, Beischel LS, Johnson JP, Shaffer LG (2002) A case of segmental paternal isodisomy of chromosome 14. Hum Genet 110:251–256
Cox H, Bullman H, Temple IK (2004) Maternal UPD(14) in the patient with a normal karyotype: clinical report and a systematic search for cases in samples sent for testing for Prader–Willi syndrome. Am J Med Genet 127A:21–25
Dib C, Faure S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 380:152–154
Eggermann T, Mergenthaler S, Eggermann K, Albers A, Linnemann K, Fusch C, Ranke MB, Wollmann HA (2001) Identification of interstitial maternal uniparental disomy (UPD) (14) and complete maternal UPD (20) in a cohort of growth retarded patients. J Med Genet 38:86–89
Engel E (1998) Uniparental disomies in unselected populations. Am J Hum Genet 63:962–966
Ferguson-Smith AC, Cattanach BM, Barton SC, Beechey CV, Surani MA (1991) Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature 351:667–670
Fritz B, Aslan M, Kalscheuer V, Ramsing M, Saar K, Fuchs B, Rehder H (2001) Low incidence of UPD in spontaneous abortions beyond the 5th gestational week. Eur J Hum Genet 9:910–916
Fujimoto M, Kantaputra PN, Ikegawa S, Fukushima Y, Sonta S, Matsuo M, Ishida T, Matsumoto T, Kondo S, Tomita H, Deng HX, D’urso M, Rinaldi MM, Ventruto V, Takagi T, Nakamura Y, Niikawa N (1998) The gene for mesomelic dysplasia Kantaputra type is mapped to chromosome 2q24–q32. J Hum Genet 43:32–36
Georgiades P, Watkins M, Surani MA, Ferguson-Smith AC (2000) Parental origin-specific developmental defects in mice with uniparental disomy for chromosome 12. Development 127:4719–4728
Gill TJ III (1986) Immunological and genetic factors influencing pregnancy and development. Am J Reprod Immunol Microbiol 10:116–120
Ginsburg C, Fokstuen S, Schinzel A (2000) The contribution of uniparental disomy to congenital development defects in children born to mothers at advanced childbearing age. Am J Med Genet 95:454–460
Hannula K, Lipsanen-Nyman M, Kontiokari T, Kere J (2001) A narrow segment of maternal uniparental disomy of chromosome 7q31-qter in Silver–Russell syndrome delimits a candidate gene region. Am J Hum Genet 68:247–253
Harrison KJ, Allingham-Hawkins DJ, Hummel J, Meschin WS, Cox DW, Costa TM, Mak-Tam E, Teshima IF, Kamel-Reid S, Winsor EJT (1998) Risk of uniparental disomy in Robertsonian translocation carriers: identification of UPD 14 in a small cohort. Am J Hum Genet (Suppl) 63:51
Hassold T, Chen N, Funkhouser J, Jooss T, Manuel B, Matsuura J, Matsuyama A, Wilson C, Yamane JA, Jacobs PA (1980) A cytogenetic study of 1,000 spontaneous abortions. Ann Hum Genet 44:151–178
Henry I, Bonaiti-Pellie C, Chehensse V, Beldjord C, Schwartz C, Utermann G, Junien C (1991) Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature 351:665–667
Hook EB, Hamerton JL (1977) The frequency of chromosomal abnormalities detected in consecutive newborn studies-differences between studies-results by sex and by severity of phenotypic involvement. In: Hook EB, Porter IH (eds) Population Cytogenetics: Studies in Humans. Academic Press, New York San Francisco London, pp 79–92
Hordijk R, Wierenga H, Scheffer H, Leegte B, Hofstra RM, Stolte-Dijkstra I (1999) Maternal uniparental disomy for chromosome 14 in a boy with a normal karyotype. J Med Genet 36:782–785
Johnston KM, Baker JC, Egli CA, McCaskill C, Shaffer LG (1996) Maternal uniparental isodisomy of chromosome 2 in a child with growth retardation, hypospadias and a cytogenetic abnormality. Am J Hum Genet (Suppl) 59:A95
Kaider AS, Kaider BD, Janowicz PB, Roussev RG (1999) Immunodiagnostic evaluation in women with reproductive failure. Am J Reprod Immunol 42:335–346
Kajii T, Ferrier A, Niikawa N, Takahara H, Ohama K, Avirachan S (1980) Anatomic and chromosomal anomalies in 639 spontaneous abortuses. Hum Genet 55:87–98
Kalousek DK, Langlois S, Barrett I, Yam I, Wilson DR, Howard-Peebles PN, Johnson MP, Giorgiutti E (1993) Uniparental disomy for chromosome 16 in humans. Am J Hum Genet 52:8–16
Katahira M, Kayashima T, Kishino T, Niikawa N (2002) Maternal uniparental disomy for chromosome 14 with diabetes mellitus. Intern Med 41:717–721
Kondo Y, Tsukishiro S, Tanemura M, Sugiura-Ogasawara M, Suzumori K, Sonta S (2004) Maternal uniparental disomy of chromosome 16 in a case of spontaneous abortion. J Hum Genet 49:177–181
Kotzot D, Schmitt S, Bernasconi F, Robinson WP, Lurie IW, Ilyina H, Mehes K, Hamel BC, Otten BJ, Hergersberg M, Hamel BC, Otten BJ, Hergersberg M (1995) Uniparental disomy 7 in Silver–Russell syndrome and primordial growth retardation. Hum Mol Genet 4:583–587
Kotzot D, Balmer D, Baumer A, Chrzanowska K, Hamel BC, Ilyina H, Krajewska-Walasek M, Lurie IW, Otten BJ, Schoenle E, Tariverdian G, Schinzel A (2000) Maternal uniparental disomy 7—review and further delineation of the phenotype. Eur J Pediatr 159:247–256
Kurosawa K, Sasaki H, Sato Y, Yamanaka M, Shimizu M, Ito Y, Okuyama T, Matsuo M, Imaizumi K, Kuroki Y, Nishimura G (2002) Paternal UPD14 is responsible for a distinctive malformation complex. Am J Med Genet 110:268–272
Ledbetter DH, Engel E (1995) Uniparental disomy in humans: development of an imprinting map and its implications for prenatal diagnosis. Hum Mol Genet 4:1757–1764
Mansfield DC, Brown AF, Green DK, Carothers AD, Morris SW, Evans HJ, Wright AF (1994) Automation of genetic linkage analysis using flourescent microsatellite markers. Genomics 24:225–233
Manzoni MF, Pramparo T, Stroppolo A, Chiaino F, Bosi E, Zuffardi O, Carrozzo R (2000) A patient with maternal chromosome 14 UPD presenting with a mild phenotype and MODY. Clin Genet 57:406–408
Martin RA, Sabol DW, Rogan PK (1999) Maternal uniparental disomy of chromosome 14 confined to an interstitial segment (14q23–14q24.2). J Med Genet 36:633–636
McGowan KD, Weiser JJ, Horwitz J, Berend SA, McCaskill C, Sutton VR, Shaffer LG (2002) The importance of investigating for uniparental disomy in prenatally identified balanced acrocentric rearrangements. Prenat Diagn 22:141–143
Miyoshi O, Hayashi S, Fujimoto M, Tomita H, Sohda M, Niikawa N (1998) Maternal uniparental disomy for chromosome 14 in a boy with intrauterine growth retardation. J Hum Genet. 43:138–142
Morison IM, Reeve AE (1998) A catalogue of imprinted genes and parent-of-origin effects in humans and animals. Hum Mol Genet 7:1599–1609
Nicholls RD, Knepper JL (2001) Genome organization, function, and imprinting in Prader–Willi and Angelman syndromes. Annu Rev Genomics Hum Genet 2:153–175
Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M (1989) Genetic imprinting suggested by maternal heterodisomy in non-deletion Prader–Willi syndrome. Nature 342:281–285
Offiah AC, Cornette L, Hall CM (2003) Paternal uniparental disomy 14: introducing the “coat-hanger” sign. Pediatr Radiol 33:509–512
Papenhausen PR, Mueller OT, Johnson VP, Sutcliffe M, Diamond TM, Kousseff BG (1995) Uniparental isodisomy of chromosome 14 in two cases: an abnormal child and a normal adult. Am J Med Genet 59:271–275
Ralph A, Scott F, Tiernan C, Caubere M, Kollegger S, Junio J, Roberts C, Ewen K, Slater HR (1999) Maternal uniparental isodisomy for chromosome 14 detected prenatally. Prenat Diagn 19:681–684
Robinson WP, Dutly F, Nicholls RD, Bernasconi F, Penaherrera M, Michaelis RC, Abeliovich D, Schinzel AA (1998) The mechanisms involved in formation of deletions and duplications of 15q11–q13. J Med Genet 35:130–136
Sanlaville D, Aubry MC, Dumez Y, Nolen MC, Amiel J, Pinson MP, Lyonnet S, Munnich A, Vekemans M, Morichon-Delvallez N (2000) Maternal uniparental heterodisomy of chromosome 14: chromosomal mechanism and clinical follow up. J Med Genet 37:525–528
Shaffer LG, McCaskill C, Egli CA, Baker JC, Johnston KM (1997) Is there an abnormal phenotype associated with maternal isodisomy for chromosome 2 in the presence of two isochromosomes? Am J Hum Genet 61:461–462
Shaffer LG, McCaskill C, Adkins K, Hassold TJ (1998) Systematic search for uniparental disomy in early fetal losses: the results and a review of the literature. Am J Med Genet 79:366–372
Smith MJ, Creasy MR, Clarke A, Upadhyaya M (1998) Sex ratio and absence of uniparental disomy in spontaneous abortions with a normal karyotype. Clin Genet 53:258–261
Splitt MP, Goodship JA (1997) Another case of maternal uniparental disomy chromosome 14 syndrome. Am J Med Genet 72:239–240
Temple IK, James RS, Crolla JA, Sitch FL, Jacobs PA, Howell WM, Betts P, Baum JD, Shield JP (1995) An imprinted gene(s) for diabetes? Nat Genet 9:110–112
Tomkins DJ, Roux AF, Waye J, Freeman VC, Cox DW, Whelan DT (1996) Maternal uniparental isodisomy of human chromosome 14 associated with a paternal t(13q14q) and precocious puberty. Eur J Hum Genet 4:153–159
Walter CA, Shaffer LG, Kaye CI, Huff RW, Ghidoni PD, McCaskill C, McFarland MB, Moore CM (1996) Short-limb dwarfism and hypertrophic cardiomyopathy in a patient with paternal isodisomy 14: 45,XY,idic(14)(p11). Am J Med Genet 65:259–265
Wang JC, Passage MB, Yen PH, Shapiro LJ, Mohandas TK (1991) Uniparental heterodisomy for chromosome 14 in a phenotypically abnormal familial balanced 13/14 Robertsonian translocation carrier. Am J Hum Genet 48:1069–1074
Warburton D, Stein Z, Kline S, Susser M (1980) Chromosome abnormalities in spontaneous abortions: data from the New York City study. In: Porter IH, Hook EB (eds) Human embryonic and fatal death. Academic Press, New York, pp 261–268
Webb AL, Sturgiss S, Warwicker P, Robson SC, Goodship JA, Wolstenholme J (1996) Maternal uniparental disomy for chromosome 2 in association with confined placental mosaicism for trisomy 2 and severe intrauterine growth retardation. Prenat Diagn 16:958–962
Acknowledgments
The authors would like to thank Professor Yusuke Nakamura of the Institute of Medical Sciences, University of Tokyo, Tokyo, Japan, for proffering oligonucleotide primers capable of detecting many polymorphisms of many microsatellite markers. This work was supported, in part, by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (Nos. 13470356 and 16390481), and a Health Sciences Research Grant for Research on Human Genome (H10-Genome-008) from the Ministry of Health, Labor and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukishiro, S., Li, Q.Y., Tanemura, M. et al. Paternal uniparental disomy of chromosome 14 and unique exchange of chromosome 7 in cases of spontaneous abortion. J Hum Genet 50, 112–117 (2005). https://doi.org/10.1007/s10038-005-0229-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10038-005-0229-9
Keywords
This article is cited by
-
NLRP7 variants in spontaneous abortions with multilocus imprinting disturbances from women with recurrent pregnancy loss
Journal of Assisted Reproduction and Genetics (2021)