Abstract
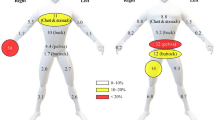
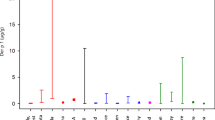
Grapevine is a vulnerable crop to several fungal diseases often requiring the use of ethylenebisdithiocarbamate (EBDC) fungicides, such as mancozeb. This fungicide has been reported to have goitrogenic, endocrine disrupting, and possibly immunotoxic effects. The aim of this study was to assess workers’ exposure in two scenarios of mancozeb application and analyse the main determinants of exposure in order to better understand their mechanism of influence. Environmental monitoring was performed using a modified Organisation for Economic Co-operation and Development (OECD) “patch” methodology and by hand-wash collection, while mancozeb’s metabolite, ethylenethiourea (ETU), was measured in 24-h preexposure and postexposure urine samples. Liquid chromatography–mass spectrometry was used for determination of mancozeb and ETU in different kinds of samples. Closed tractor use resulted in 40 times lower potential exposure compared with open tractor. Coveralls reduced skin exposure 4 and 10 times in case of open and closed tractors, respectively. Gloves used during application resulted in 10 times lower hand exposure in open but increased exposure in closed tractors. This study has demonstrated that exposure to mancozeb is low if safe occupational hygiene procedures are adopted. ETU is confirmed as suitable biological marker of occupational exposure to mancozeb, but the absence of biological exposure limits significantly reduces the possibility to interpret biological monitoring results in occupationally exposed workers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Maroni M, Colosio C, Ferioli A, Fait A . Biological monitoring of pesticide exposure: a review. Introduction. Toxicology 2000; 143: 1–118.
Vergova M, Jablonica A, Janota S . Occupational exposure to mancozeb in employees in the production of Novozir Mn 80. Pracov Lek 1988; 40: 425–430.
Smith DM . Ethylene thiourea: thyroid function in two groups of exposed workers. Br J Ind Med 1984; 41: 362–366.
Cecconi S, Paro R, Rossi G, Macchiarelli G . The effects of the endocrine disruptors dithiocarbamates on the mammalian ovary with particular regard to mancozeb. Curr Pharmaceut Des 2007; 13: 2989–3004.
Bisson M, Hontela A . Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro. Toxicol Appl Pharmacol 2002; 180: 110–117.
Panganiban L, Cortes-Maramba N, Dioquino C, Suplido ML, Ho H, Francisco-Rivera A et al. Correlation between blood ethylenethiourea and thyroid gland disorders among banana plantation workers in the Philippines. Environ Health Perspect 2004; 112: 42.
Colosio C, Fustinoni S, Corsini E, Bosetti C, Birindelli S, Boers D et al. Changes in serum markers indicative of health effects in vineyard workers following exposure to the fungicide mancozeb: an Italian study. Biomarkers 2007; 12: 574–588.
Corsini E, Viviani B, Birindelli S, Gilardi F, Torri A, Codecà I et al. Molecular mechanisms underlying mancozeb-induced inhibition of TNF-alpha production. Toxicol Appl Pharmacol 2006; 212: 89–98.
Corsini E, Liesivuori J, Vergieva T, Van Loveren H, Colosio C . Effects of pesticide exposure on the human immune system. Hum Exp Toxicol 2008; 27: 671–680.
Somerville L . The metabolism of fungicides. Xenobiotica 1986; 16: 1017–1030.
Colosio C, Fustinoni S, Birindelli S, Bonomi I, De Paschale G, Mammone T et al. Ethylenethiourea in urine as an indicator of exposure to mancozeb in vineyard workers. Toxicol Lett 2002; 134: 133–140.
Kurttio P, Vartiainen T, Savolainen K . Environmental and biological monitoring of exposure to ethylenebisdithiocarbamate fungicides and ethylenethiourea. Br J Ind Med 1990; 47: 203–206.
EU Regulation. No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. EU, Brussels, Belgium 2009.
EU Commission Document. European Commission Health & Consumer Protection Directorate-General Directorate D - Food Safety: Production and distribution chain Unit D.3 - Chemicals, contaminants and pesticides Mancozeb SANCO/4058/2001 - rev. 4.4 July 2009 (http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.detail&language=EN&selectedID=1531).
Colosio C, Visentin S, Birindelli S, Campo L, Fustinoni S, Mariani F et al. Reference values for ethylenethiourea in urine in Northern Italy: results of a pilot study. Toxicol Lett 2006; 162: 153–157.
Arbuckle TE, Burnett R, Cole D, Teschke K, Dosemeci M, Bancej C et al. Predictors of herbicide exposure in farm applicators. Int Arch Occup Environ Health 2002; 75: 406–414.
Damalas CA, Koutroubas SD . Farmers’ exposure to pesticides: toxicity types and ways of prevention. Toxics 2016; 4: 1–10.
Coble J, Arbuckle T, Lee W, Alavanja M, Dosemeci M . The validation of a pesticide exposure algorithm using biological monitoring results. J Occup Environ Hygiene 2005; 2: 194–201.
Brouwer DH, Marquart H, van Hemmen JJ . Proposal for an approach with default values for the protection offered by PPE, under European new or existing substance regulations. Ann Occup Hygiene 2001; 45: 543–553.
Gomes J, Lloyd OL, Revitt DM . The influence of personal protection, environmental hygiene and exposure to pesticides on the health of immigrant farm workers in a desert country. Int Arch Occup Environ Health 1999; 72: 40–45.
Mandic-Rajcevic S, Rubino FM, Vianello G, Fugnoli L, Polledri E, Mercadante R et al. Dermal exposure and risk assessment of tebuconazole applicators in vineyards. Med Lav 2015; 106: 294–315.
OECD. OECD Environment Directorate. Environmental Health and Safety Publications. Series on Testing and Assessment. No. 9. Guidance Document for the Conduct of Studies of occupational Exposure to Pesticides During Agricultural Application. Paris 1997. (http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ocde/gd(97)148&doclanguage=en).
Mosteller RD . Simplified calculation of body-surface area. N Engl J Med 1987; 317: 1098.
Vitali M, Protano C, Del Monte A, Ensabella F, Guidotti M . Operative modalities and exposure to pesticides during open field treatments among a group of agricultural subcontractors. Arch Environ Contam Toxicol 2009; 57: 193–202.
Aprea C, Terenzoni B, De Angelis V, Sciarra G, Lunghini L, Borzacchi G et al. Evaluation of skin and respiratory doses and urinary excretion of alkylphosphates in workers exposed to dimethoate during treatment of olive trees. Arch Environ Contam Toxicol 2005; 48: 127–134.
Flack S, Goktepe I, Ball LM, Nylander-French LA . Development and application of quantitative methods for monitoring dermal and inhalation exposure to propiconazole. J Environ Monit 2008; 10: 336–344.
Lima CS, Nunes-Freitas AL, Ribeiro-Carvalho A, Filgueiras CC, Manhaes AC, Meyer A et al. Exposure to methamidophos at adulthood adversely affects serotonergic biomarkers in the mouse brain. Neurotoxicology 2011; 32: 718–724.
R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. 2012.
Aprea MC . Environmental and biological monitoring in the estimation of absorbed doses of pesticides. Toxicol Lett 2012; 210: 110–118.
Rubino FM, Mandic-Rajcevic S, Ariano E, Alegakis A, Bogni M, Brambilla G et al. Farmers’ exposure to herbicides in North Italy: assessment under real-life conditions in small-size rice and corn farms. Toxicol Lett 2012; 210: 189–197.
Tsakirakis AN, Kasiotis KM, Charistou AN, Arapaki N, Tsatsakis A, Tsakalof A et al. Dermal & inhalation exposure of operators during fungicide application in vineyards. Evaluation of coverall performance. Sci Total Environ 2014; 470–471: 282–289.
Lundehn J, Westphal D, Kieczka H . Uniform principles for safeguarding the health of applicators of plant protection products. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft 1992; 227: 60–112.
van Hemmen JJ . EUROPOEM, a predictive occupational exposure database for registration purposes of pesticides. Appl Occup Environ Hygiene 2001; 16: 246–250.
Fenske RA, Blacker AM, Hamburger SJ, Simon GS . Worker exposure and protective clothing performance during manual seed treatment with lindane. Arch Environ Contam Toxicol 1990; 19: 190–196.
Protano C, Guidotti M, Vitali M . Performance of different work clothing types for reducing skin exposure to pesticides during open field treatment. Bull Environ Contam Toxicol 2009; 83: 115–119.
Machera K, Tsakirakis A, Charistou A, Anastasiadou P, Glass CR . Dermal exposure of pesticide applicators as a measure of coverall performance under field conditions. Ann Occup Hygiene 2009; 53: 573–584.
Hines CJ, Deddens JA, Tucker SP, Hornung RW . Distributions and determinants of pre-emergent herbicide exposures among custom applicators. Ann Occup Hygiene 2001; 45: 227–239.
Yoshida K, Fuzesi I, Suzan M, Nagy L . Measurements of surface contamination of spray equipment with pesticides after various methods of application. J Environ Sci Health Part B Pesticides Food Contam Agric Wastes 1990; 25: 169–183.
Sanderson WT, Ringenburg V, Biagini R . Exposure of commercial pesticide applicators to the herbicide alachlor. Am Ind Hygiene Assoc J 1995; 56: 890–897.
Colosio C, Rubino FM, Alegakis A, Ariano E, Brambilla G, Mandic-Rajcevic S et al. Integration of biological monitoring, environmental monitoring and computational modelling into the interpretation of pesticide exposure data: introduction to a proposed approach. Toxicol Lett 2012; 213: 49–56.
Baldi I, Lebailly P, Jean S, Rougetet L, Dulaurent S, Marquet P . Pesticide contamination of workers in vineyards in France. J Expo Sci Environ Epidemiol 2006; 16: 115–124.
Unitd States Environmental Protection Agency. Office of Health and Environmental Assessment. Dermal Exposure Assessment: Principles and Applications. Interim Report. EPA/600/8-91/011B. January 1992. (https://rais.ornl.gov/documents/DERM_EXP.PDF).
Frasch HF, Dotson GS, Bunge AL, Chen CP, Cherrie JW, Kasting GB et al. Analysis of finite dose dermal absorption data: implications for dermal exposure assessment. J Expo Sci Environ Epidemiol 2014; 24: 65–73.
Acknowledgements
We acknowledge the support of the Italian Institute for Insurance of Occupational Diseases and Accidents (INAIL)—Session of the Region of Lombardy, which funded this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mandic-Rajcevic, S., Rubino, F., Ariano, E. et al. Environmental and biological monitoring for the identification of main exposure determinants in vineyard mancozeb applicators. J Expo Sci Environ Epidemiol 28, 289–296 (2018). https://doi.org/10.1038/jes.2017.14
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/jes.2017.14