Abstract
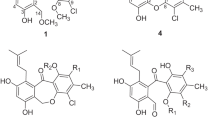
Two new meroterpenes, phenylpyropenes E (1) and F (2), together with seven known phenylpyropenes (3–5) and pyripyropenes (6–9) were isolated from the marine-derived fungus Penicillium concentricum ZLQ-69. Their structures including the absolute configurations were elucidated using a combination of spectroscopic methods and electronic circular dichroism calculation. Bioactivity evaluation showed that compounds 1 and 4 were cytotoxic to the MGC-803 cell line with IC50 values of 19.1 and 13.6 μM, respectively.
Similar content being viewed by others
Introduction
Pyripyropenes and phenylpyropenes are subclasses of meroterpenes existing in the filamentous fungi genuses Aspergillus and Penicillium. These compounds are biogenetically derived from a hybrid of polyketide and terpenoid. Their structures consist of three parts: a pyridine/phenyl ring, an α-pyrone and a sesquiterpene motif. After they were first isolated in 1994, 19 pyripyropenes were shown to be effective as acyl-CoA/cholesterol acyltransferase (ACAT) inhibitors and are predicted to be useful in the prevention and treatment of hypercholesterolemia and atherosclerosis.1, 2, 3, 4, 5 Because of their structural novelty and promising biological properties, pyripyropenes are attractive targets for synthetic and biological study, with over 300 chemical derivatives synthesized for studies of ACAT inhibitory activity and structure-activity relationships.6, 7, 8, 9, 10, 11, 12, 13, 14, 15 In addition, pyripyropenes have been found to have anticancer16, 17 and anti-insect activity.18, 19 Compared with the comprehensive study of pyripyropenes, much less research has been conducted on the discovery and investigation of phenylpyropenes. Until now, only four phenylpyropenes A–D that displayed ACAT inhibitory activity have been reported.20, 21, 22 Further research showed that phenylpyropene C can inhibit the inducible expression of the pro-inflammatory enzymes COX-2 and iNOS and the cytokine TNF-α in various cell lines.23, 24
In our ongoing research on the bioactive secondary metabolites of marine-derived fungi, the strain Penicillium concentricum ZLQ-69 (isolated from the water samples taken from the coast of Bohai Sea in Binzhou, Shandong Province, China) displayed interesting HPLC-UV profiles and cytotoxicity. Further chemical investigation of this extract led to the discovery of two new phenylpyropenes, named phenylpyropenes E (1) and F (2), as well as seven known phenylpyropenes (3–5) and pyripyropenes (6–9) (Figure 1). In this paper, we report the isolation and structural determination of the new compounds (1 and 2), and the cytotoxicity of five phenylpyropenes (1–5).
Results and Discussion
Phenylpyropene E (1) was obtained as a pale yellow amorphous powder. The molecular formula C30H36O9 was determined by the positive HRESIMS (m/z 541.2448 [M+H]+; calcd, 541.2432), indicating 13 degrees of unsaturation. The 1D NMR data (Table 1) showed 30 carbon signals that were classified by DEPT and HMQC spectra as five methyl groups, four methylene groups, eleven methine groups including three that were oxygenated and six sp2 groups and ten quaternary carbons including five ester or enol ketone groups and two sp2 groups. The 1H- and 13C-NMR data (Table 1) of 1 were similar to those reported for phenylpyropene A.20 The difference was the lack of an acetyl group and the appearance of an additional hydroxy group in 1. The functional alcohol group was located at C-3, as evidenced by the up-field shifts of H-3 (δH 3.46) and C-3 (δC 71.4) in 1 compared with phenylpyropene A (δH 4.72 and δC 73.6),20 which could be confirmed by the COSY correlations from H-1 to H-3 as well as the key HMBC correlations from H-3 (δH 3.46) to C-1 (δC 36.6) and C-4 (δC 41.8). The planar structure of 1 was thus established and named phenylpyropene E.
Phenylpyropene F (2) was isolated as a pale yellow amorphous powder with the molecular formula C28H34O6 assigned by the positive HRESIMS (m/z 489.2998 [M+Na]+; calcd, 489.2986). Similarly, 1D NMR data (Table 1) between compound 2 and compound 4 indicated that they shared the same structural skeleton.21 The main difference was the replacement of the methylene group (CH2-11) in 4 by a hydroxylated methine group in 2, indicated by a chemical shift of C-11 (δC 59.1) and the COSY correlation between H-9 and H-11, as well as the HMBC correlation from H-11 (δH 4.94) to C-8 (δC 81.8), C-10 (δC 37.4), C-12 (δC 102.2) and C-13 (δC 164.0).
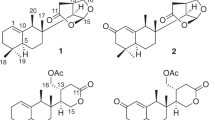
The relative configurations of 1 and 2 were determined by the NOESY experiments (Figure 2). In the NOESY spectrum of 1, correlations were observed between H3-17 and H3-18 and H3-19, which indicated that these protons were on the same face of the molecule, whereas correlations between H-5 and H-3, H-7 and H-9 suggested that they were on another face. In addition, the molecular modeling study and NOESY correlations between H-3 and H2-20, H-9 and H-11 showed that H-20 and H-11 were equatorial. All of these findings verified the relative configuration of compound 1, which was identical to pyripyropene A.25 The relative configuration of 2 was determined to be the same as 1 by the NOESY spectrum. The absolute configurations of 1 and 2 were determined by comparing their electronic circular dichroism (ECD) curves with that of the calculated curve using the matrix model. The ECD spectrum of the simplified model compound a, the deacetyl analog of 1, was calculated at the B3LYP/6-31+G(d) level. The experimental ECD spectra of 1 and 2 were in strong agreement with the calculated model compound a (Figure 3). Thus, the absolute configuration of 1 was determined to be 3S, 4R, 5R, 7S, 8S, 9S, 10S,11R, which was the same as that of pyripyropene A,25 whereas the absolute configuration of 2 was determined to be 3S, 5R, 8R, 9S, 10S, 11R.
The structures of known compounds were determined by comparing their spectroscopic data with that reported in the literature, and these compounds were finally identified as phenylpyropenes B–D (3–5)20, 21, 22 and pyripyropenes A, B, E and O (6–9).2, 3, 4
The phenylpyropenes (1–5) were tested for in vitro cytotoxic activity on three different human cancer cell lines (lung adenocarcinoma A549, gastric cancer MGC-803 and leukemia HL-60) using the MTT method. The results showed that only compounds 1 and 4 have moderate cytotoxicity against the MGC-803 cell line with IC50 values of 19.1 and 13.6 μM, respectively. In previous reports, pyripyropenes showed no cytoxicity against five human cancer cell lines (IC50>100 μM).26
In conclusion, two new phenylpyropenes, E and F (1 and 2), were isolated from P. concentricum ZLQ-69. The absolute configurations of these phenylpyropenes were deduced by ECD calculations and phenylpyropenes E (1) showed moderate cytotoxicity. The biosynthetic procedure of phenylpyropenes was believed to be similar to that of pyripyropenes, which have already been described.27, 28 The differences between phenylpyropenes and pyripyropenes might be a consequence of their different starting units (benzoic acid and nicotinic acid, respectively). In this study, we isolated a fungal strain (P. concentricum ZLQ-69) that can simultaneously produce both phenylpyropenes and pyripyropenes, similar to Penicillium griseofulvum and Aspergillus similanensis as previously reported.5, 22
Experimental procedure
General
Optical rotations were obtained on a JASCO P-1020 digital polarimeter (JASCO, Tokyo, Japan). UV spectra were recorded on a Beckman DU 640 spectrophotometer (Beckman Ltd, Shanghai, China). IR spectra were recorded on a Nicolet NEXUS 470 spectrophotometer (Thermo Scientific, Beijing, China) in KBr disks. CD spectra were measured on a JASCO J-715 spectropolarimeter (JASCO, Tokyo, Japan). NMR spectra were recorded on JEOL JUM-ECP 600 (JEOL, Beijing, China) and Agilent 500 MHz DD2 spectrometers (Agilent, Beijing, China) using tetramethylsilane as an internal standard, and the chemical shifts were recorded as δ values. ESIMS was measured on a Micromass Q-TOF Ultima Global GAA076 LC mass spectrometer (Waters Asia Pacific headquarters, Tokyo, Japan). HRESIMS spectra were measured on a Micromass EI-4000 (Autospec-Ultima-TOF, Waters, Shanghai, China). TLC and column chromatographywere performed either on plates precoated with silica gel GF254 (10–40 μm) or over silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China). Size-exclusion chromatography was performed using the Sephadex LH-20 (GE Healthcare, Bio-Sinences Corp, Piscataway, NJ, USA). Semi-preparative HPLC was performed using an ODS column (YMC-Pack ODS-A, 10 × 250 mm, 5 μm).
Fungal resource
The marine-derived fungus P. concentricum ZLQ-69 was isolated from water sample taken from the coast of the Bohai Sea in Binzhou, Shandong Province, China. The isolate was identified by ITS-rDNA sequence analysis. The ITS1-5.8S-ITS2 rDNA sequence of the fungus ZLQ-69 was submitted to GenBank with the accession number KM386990. The voucher specimen has been deposited in our laboratory at −80 °C. The working strain was prepared on potato dextrose agar and stored at 4 °C.
Fermentation and extraction
The fungus ZLQ-69 was cultured stationarily at 28 °C for 30 days in 100 Erlenmeyer flasks (1 l) containing 60 g of rice and 20 ml of natural seawater. At the end of the incubation period, the mycelia and medium were homogenized, extracted with MeOH-H2O (5:1, v/v; 120 ml each flask) and concentrated under reduced pressure to produce an aqueous solution. The aqueous solution was extracted three times with equal volumes of EtOAc. The EtOAc solution was concentrated under reduced pressure to produce the organic extract (20.5 g).
Purification
The EtOAc extract (20.5 g) was subjected to vacuum liquid chromatography over a silica gel column using a gradient elution with petroleum ether-CHCl3-MeOH to produce 10 fractions (fractions 1–10). Fraction 3 was chromatographed on a silica gel column (petroleum ether/EtOAc, 2:1) and by semi-preparative HPLC (75% MeOH/H2O, 3 ml min−1) to produce compound 4 (tR 15 min; 9 mg). Fraction 5 was separated by Sephadex LH-20 chromatography (MeOH) to produce four subfractions (Fraction 5.1–5.4). Fraction 5.2 was further purified by semi-preparative HPLC (70% MeOH/H2O, 3 ml min−1) to produce compound 1 (tR 13 min; 19 mg) and compound 2 (tR 16.5 min; 10 mg). Further purification of Fraction 5.3 by preparative HPLC (80% MeOH/H2O, 3 ml min−1) produced compounds 8 (tR 14 min; 9 mg) and 9 (tR 17 min; 4.5 mg). Fraction 7 was subjected to Sephadex LH-20 chromatography (CHCl3/MeOH, 1:1) and then purified by semi-preparative HPLC (70% MeOH-H2O, 3 ml min−1) to produce compounds 6 (tR 9 min; 27 mg) and 7 (tR 19 min; 11 mg). Fraction 10 was subjected to Sephadex LH-20 chromatography (MeOH) and then further purified by semi-preparative HPLC (60% MeOH/H2O, 3 ml min−1) to produce compounds 3 (tR 15 min; 17 mg) and 5 (tR 19 min; 20 mg).
Phenylpyropene E ( 1). Light yellow powder; [α]25D +86.1 (c 0.1, CHCl3); UV (MeOH) λmax (log ɛ) 211 (3.75), 245 (2.74), 312 (1.88) nm; CD (0.9 × 10−3M, MeOH) λmax (Δɛ) 315 (−0.24), 267 (1.92), 231 (11.23), 201 (13.57) nm; 1H- and 13C-NMR data, see Table 1; HRESIMS m/z 541.2448 [M+H]+ (calcd. for C30H37O9, 541.2432).
Phenylpyropene F ( 2). Light yellow powder; [α]25D +52.4 (c 0.1, CHCl3); UV (MeOH) λmax (log ɛ) 211 (3.56), 245 (2.78), 310 (1.90) nm; CD (1.1 × 10−3 M, MeOH) λmax (Δɛ) 267 (2.06), 231 (17.11), 201 (17.14) nm; 1H- and 13C-NMR data, see Table 1; HRESIMS m/z 489.2998 [M+Na]+ (calcd. for C28H34O6Na, 489.2986).
Cytotoxicity assay
The cytotoxic activity of compounds 1–5 was evaluated by the MTT method.29 In this assay, the A-549, MGC-803 and HL-60 cell lines were grown in RPMI-1640 supplemented with 10% FBS under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. Cell suspensions (200 μl) at a density of 5 × 104 cell ml−1 were plated in 96-well microtiter plates and incubated for 24 h. Then, 2 μl of the test solutions in DMSO were added to each well and further incubated for 72 h. The MTT solution (20 μl, 5 mg ml−1 in IPMI-1640 medium) was then added to each well and incubated for 4 h. Old medium containing MTT (150 ml) was gently replaced by DMSO and pipetted to dissolve any formazan crystals formed. The absorbance was determined by a Spectra Max Plus (Molecular Devices Corp, Sunnyvale, CA, USA) plate reader at 540 nm. Doxorubicin was used as the positive control (IC50 values 0.64, 0.37 and 0.029 μM).
Computation
Conformational searches were run employing the “systematic” procedure implemented in Spartan’14, using Merck molecular force field.30 All Merck molecular force field minima were re-optimized with density functional thoery (DFT) calculations at the B3LYP/6-31+G(d) level using the Gaussian09 program.31 The geometry was optimized starting from various initial conformations, with vibrational frequency calculations confirming the presence of minima. Time-dependent DFT calculations were performed on the lowest-energy conformations (>5% population) for each configuration using 30 excited states and a polarizable continuum model for chloroform. ECD spectra were generated using the program SpecDis by applying a Gaussian band shape with 0.26 eV width, from dipole-length rotational strengths.32 The dipole velocity forms yielded negligible differences. The spectra of the conformers were combined using the Boltzmann weighting, with the lowest-energy conformations accounting for ~99% of the weights. The calculated spectrum was blue-shifted by 10 nm to facilitate comparison to the experimental data.
Accession codes
References
Tomoda, H., Kim, Y. K., Nishida, H., Masuma, R. & Omura, S. Pyripyropenes, noval inhibitors of Acyl-CoA: cholesterol acyltransferase produced by Aspergillus fumigatus. I. Production, isolation and biological properties. J. Antibiot. 47, 148–153 (1994).
Kim, Y. K. et al. Pyripyropenes, noval inhibitors of Acyl-CoA: cholesterol acyltransferase produced by Aspergillus fumigatus. II. Structure elucidation of pyripyropenes A, B, C and D. J. Antibiot. 47, 154–162 (1994).
Tomoda, H. et al. Pyripyropenes, noval ACAT inhibitors produced by Aspergillus fumigatus. III. Structure elucidation of pyripyropenes E to L. J. Antibiot. 48, 495–503 (1995).
Tomoda, H. et al. Pyripyropenes, noval ACAT inhibitors produced by Aspergillus fumigatus. IV. Structure elucidation of pyripyropenes M to R. J. Antibiot. 49, 292–298 (1996).
Prompanya, C. et al. New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. nov. KUFA 0013. Mar. Drugs 12, 5160–5173 (2014).
Obata, R., Sunazuka, T., Li, Z., Tomoda, H. & Omura, S. Structure-activity relationships of pyripyropenes, fungal acyl-CoA: cholesterol acyltransferase inhibitors. J. Antibiot. 48, 749–750 (1995).
Obata, R., Sunazuka, T., Tomoda, H., Harigaya, Y. & Omura, S. Chemical modification and structure-activity relationships of pyripyropenes; Potent orally bioavailable inhibitors of acyl-CoA: cholesterol O-acyltransferase (ACAT). Bioorg. Med. Chem. Lett. 5, 2683–2688 (1995).
Obata, R. et al. Chemical modification and structure-activity relationships of pyripyropenes. 1. Modification at the four hydroxyl groups. J. Antibiot. 49, 1133–1148 (1996).
Obata, R. et al. Chemical modification and structure-activity relationships of pyripyropenes. 2. 1, 11-cyclic analogs. J. Antibiot. 49, 1149–1156 (1996).
Obata, R. et al. Chemical modification and structure-activity relationships of pyripyropenes. 3. Synthetic conversion of pyridine-pyrone moiety. J. Antibiot. 50, 229–236 (1997).
Obata, R. et al. New analogs of pyripyropene family of ACAT inhibitors via α-pyrone fragmentation and γ-acylation/cyclization. Chem. Lett. 26, 935–937 (1997).
Ohshiro, T. et al. Selectivity of pyripyropene derivatives in inhibition toward acyl-CoA: cholesterol acyltransferase 2 isozyme. J. Antibiot. 61, 503–508 (2008).
Ohtawa, M. et al. Synthesis and structure–activity relationship of pyripyropene A derivatives as potent and selective acyl-CoA: cholesterol acyltransferase 2 (ACAT2) inhibitors: Part 1. Bioorg. Med. Chem. Lett. 23, 1285–1287 (2013).
Ohtawa, M. et al. Synthesis and structure–activity relationship of pyripyropene A derivatives as potent and selective acyl-CoA: cholesterol acyltransferase 2 (ACAT2) inhibitors: Part 2. Bioorg. Med. Chem. Lett. 23, 2659–2662 (2013).
Ohtawa, M. et al. Synthesis and structure–activity relationship of pyripyropene A derivatives as potent and selective acyl-CoA: cholesterol acyltransferase 2 (ACAT2) inhibitors: Part 3. Bioorg. Med. Chem. Lett. 23, 3798–3801 (2013).
Obata, R. et al. Structure-activity relationships study of pyripyropenes: reversal of cancer multidrug resistance. J. Antibiot. 53, 422–425 (2000).
Hayashi, A., Arai, M., Fujita, M. & Kobayashi, M. Pyripyropenes, fungal sesquiterpenes conjugated with alpha-pyrone and pyridine moieties, exhibits anti-angiogenic activity against human umbilical vein endothelial cells. Biol. Pharm. Bull. 32, 1261–1265 (2009).
Kimhiko, G. et al. Pest control agents containing pyripyropenes. WO2006129714 (A1) (2006).
Ryo, H., Mitsuyuki, Y., Shinji, S., Kazuhiko, O. & Masaaki, M. Novel systemic pesticide with synerigistic effect. WO2009081851 (A1) (2009).
Kwon, O. E. et al. Phenylpyropene A and B, new inhibitors of acyl-CoA: cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 55, 1004–1008 (2002).
Rho, M. C. et al. Phenylpyropene C, a new inhibitor of acyl-CoA: cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J. Antibiot. 55, 211–214 (2002).
Kim, Y. K. et al. Novel phenylpyropene D, preparation method thereof and pharmaceutical composition comprising the same. KR20130100618 (A) (2013).
Erkel, G., Rether, J. & Anke, T. S14-95, a novel inhibitor of the JAK/STAT Pathway from a Penicillium species. J. Antibiot. 56, 337–343 (2003).
Yao, Y. et al. Sporogen, S14-95, and S-curvularin, three inhibitors of human inducible nitric-oxide synthase expression isolated from fungi. Mol. Pharmacol. 63, 383–391 (2003).
Tomoda, H. et al. Relative and absolute stereochemistry of pyripyropene A, a potent, bioavailable inhibitor of Acyl-CoA: cholesterol acyltransferase (ACAT). J. Am. Chem. Soc. 116, 12097–12098 (1994).
Masi, M. et al. Fischerindoline, a pyrroloindole sesquiterpenoid isolated from Neosartorya pseudofischeri, with in vitro growth inhibitory activity in human cancer cell lines. Tetrahedron 69, 7466–7470 (2013).
Itoh, T. et al. Reconstitution of a fungal meroterpenoid biosynthesis reveals the involvement of a novel family of terpene cyclases. Nat. Chem. 2, 858–864 (2010).
Hu, J. et al. Characterization of two cytochrome P450 monooxygenase genes of the pyripyropene biosynthetic gene cluster from Penicillium coprobium. J. Antibiot. 64, 221–227 (2011).
Wu, G. W. et al. Penilactones A and B, two novel polyketides from Antarctic deep-sea derived fungus Penicillium crustosum PRB-2. Tetrahedron 68, 9745–9749 (2012).
Spartan 14, (Wavefunction Inc. Irvine, CA, USA (2013).
Frisch, M. J. et al Gaussian 09, revision B.01, Gaussian Inc., Wallingford, CT, USA, (2010).
Bruhn, T., Hemberger, Y., Schaumlöffel, A. & Bringmann, G. SpecDis, Version 1.53, University of Wuerzburg, Germany, (2011).
Acknowledgements
This work was financially supported by the Chinese National Natural Science Fund (No. 21372208, 41176120), the NSFC-Shandong Joint Fund for Marine Science Research Centers (U1406402), the National High Technology Research and Development Program of China (No. 2013AA092901), The Shandong Provincial Natural Science Fund for distinguished young scholars (JQ201422), the Program for New Century Excellent Talents in University (NCET-12-0499) and the Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (No. GD2012-D01-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Ding, Z., Zhang, L., Fu, J. et al. Phenylpyropenes E and F: new meroterpenes from the marine-derived fungus Penicillium concentricum ZLQ-69. J Antibiot 68, 748–751 (2015). https://doi.org/10.1038/ja.2015.64
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2015.64
This article is cited by
-
Saroclazines A–C, thio-diketopiperazines from mangrove-derived fungi Sarocladium kiliense HDN11-84
Archives of Pharmacal Research (2018)