Abstract
A novel abrogator of bleomycin-induced G2 arrest in Jurkat cells, habiterpenol (1), was isolated from the culture broth of Phytohabitans suffuscus 3787_5. The planar structure of 1 was elucidated by spectroscopic study (1D and 2D NMR, MS, UV and IR), and the relative stereochemistry was elucidated by ROESY experiments. Compound 1 belongs to a pentacyclic meroterpenoid having a labdan-type diterpene connecting to an indane moiety.
Similar content being viewed by others
Introduction
During the course of screening for microbial abrogators of bleomycin-induced G2 arrest in Jurkat cells, a new compound, named habiterpenol (1, Figure 1), was isolated from the culture broth of Phytohabitans suffuscus 3787_5. The taxonomy of the producing actinomycete, fermentation, isolation and biological properties of 1 are described in the accompanying paper.1 In this study, the physicochemical properties and structure elucidation of 1 are described.
Results
Physicochemical properties of habiterpenol
The physicochemical properties of habiterpenol (1) are summarized in Table 1. In UV spectra, 1 showed absorption maxima at 221, 234(sh) and 284 nm. In IR spectra, broad OH absorption near 3428 cm−1, typical C–H (CH2) stretching absorptions at 2929 and 2851 cm−1 and aromatic C–C stretch absorptions (for carbon–carbon bonds in the aromatic ring) at 1614 and 1461 cm−1 were observed.
Planar structure of habiterpenol
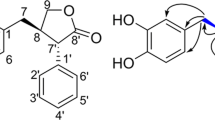
Compound 1 showed a molecular ion peak at m/z 366 [M]+ in EI-MS, and the molecular formula C26H38O was assigned on the basis of its HR-EIMS (m/z 366.2929 [M]+, Δ+0.6 mmu), indicating 8 degrees of unsaturation. The 1H and 13C NMR spectra of 1 in CDCl3 (Table 2) showed 38 proton and 26 carbon signals, which were confirmed by analysis of 2D NMR correlations. The multiplicity of the carbon signals was classified into five methyl carbons, eight sp3 methylene carbons, three sp2 methine carbons, three sp3 methine carbons, two sp2 quaternary carbons, four sp3 quaternary carbons and one sp2 oxygenated quaternary carbon by analysis of DEPT and HMQC data. The connectivity of proton and carbon atoms was established by HMQC (Table 2). As shown in Figure 2, the partial structures I–V were elucidated by 1H–1H COSY spectra and TOCSY.
The 13C–1H long-range couplings of 2J and 3J in the HMBC spectra (Figure 3) proved the presence of the following linkages: (1) The cross peaks from the sp3 methine proton H-10 (δ 0.82) to the sp3 quaternary carbon C-11 (δ 37.4) and the methyl carbon C-18 (δ 16.4), from sp3 methylene protons H2-13 (δ 1.60, 1.40) to the sp3 quaternary carbon C-15 (δ 33.3), from the methyl protons H3-18 (δ 0.73) to the sp3 methine carbon C-6 (δ 57.9), C-11 and the sp3 methylene carbon C-12 (δ 40.1), and from the methyl protons H3-19 (δ 0.78) and H3-20 (δ 0.85) to the sp3 methine carbon C-10 (δ 56.8) and the sp3 methylene carbon C-14 (δ 42.2) and C-15 suggested the presence of a 11,15,15-trimethyl-10,11-disubstituted cyclohexane (ring A) containing the partial structure I. (2) Long-range couplings from the sp3 methylene protons H2-8 (δ 1.82, 1.02) to the quaternary carbon C-7 (δ 37.4) and C-10, from the methyl protons H3-17 (δ 0.33) to C-6, C-7 and sp3 methylene carbon C-8 (δ 43.0), and from H3-18 to C-6 showed that a 7,11-dimethyl-6,7,10,11-tetrasubstituted cyclohexane ring (ring B) containing the partial structure II is attached to ring A, revealing the presence of a 7,11,15,15-tetramethyl bicyclo[4.4.0]decane moiety. (3) Long-range couplings from the sp3 methylene protons H2-1 (δ 2.94, 2.58) to the sp3 quaternary carbon C-3 (δ 46.3), from the sp3 methine proton H-2 (δ 1.72) to C-3, C-7 and the methyl carbon C-16 (δ 33.5), from the sp3 methylene protons H2-4 (δ 2.25, 1.56) to the sp3 methine carbon C-2 (δ 63.1), C-3 and C-16, from the sp3 methylene protons H2-5 (δ 1.47, 1.15) to C-3 and C-7, from the methyl protons H3-16 (δ 1.06) to C-2, C-3 and the sp3 methylene carbon C-4 (δ 34.9), and from H3-17 to C-2 showed that a 3,7-dimethyl-2,3,6,7-tetrasubstituted cyclohexane ring (ring C) containing the partial structures III and IV is attached to ring B to form a labdane-type diterpene substructure. (4) Long-range couplings from the methine proton H-2′ (δ 6.96) to the oxygenated sp2 quaternary carbon C-4′ (δ 154.2) and the sp2 quaternary carbon C-6′ (δ 153.8), from the sp2 methine proton H-3′ (δ 6.55) to C-4′, the sp2 methine carbon C-5′ (δ 107.8) and the sp2 quaternary carbon C-1′ (δ 135.9), and from the sp2 methine proton H-5′ (δ 6.54) to the sp2 methine carbon C-3′ (δ 112.6), C-4′ and C-6′ showed the presence of a 4′-oxy-1′,6′-disubstituted benzene ring (ring E) containing the partial structure V. Further, the two ortho-coupling constants (7.5 Hz) observed between aromatic protons H-2′ and H-3′ also supported that they are in the ortho position of the benzene ring. (5) This benzene ring (ring E) was connected to a labdane-type diterpene moiety by observation of the 2J and 3J cross peaks from H2-1 to C-1′ and the sp2 methine carbon C-2′ (δ 124.6), from H-2 to C-1′ and C-6′, from H-2′ to C-1 (δ 30.9), from H-5′ to C-3, and from H3-16 to C-6′ in HMBC experiments, indicating the presence of an indane moiety (Figure 3). Furthermore, the chemical shifts of C-4′ (δ 154.2) indicated that a hydroxyl group is attached to C-4′. Taking these observations into consideration, the planar structure of 1 was elucidated as shown in Figure 1, which fulfilled the molecular formula and the degrees of unsaturation.
Relative stereochemistry of habiterpenol
Compound 1 has six chiral carbons. The relative stereochemistry of 1 was elucidated by analysis of ROESY experiments as shown in Figure 4. NOEs were observed between H3-18 (δ 0.73) and H3-19 (δ 0.78), between Hax-13 (δ 1.60) and H3-19 and between Hax-13 and H3-18, indicating that they are all oriented to the same molecular face. On the other hand, NOEs were observed between H-10 (δ 0.82) and Hax-14 (δ 1.15) and between Hax-12 (δ 0.84) and Hax-14, indicating that they are all oriented to the opposite molecular face. These results strongly suggested trans-diaxal disposition of H-10 and H3-18. NOEs were also observed between Hax-9 (δ 1.26) and H3-17 (δ 0.33), between Hax-9 and H3-18 and between H3-17 and H3-18, indicating that they are all oriented to the same molecular face. On the other hand, NOEs were observed between H-6 (δ 0.85) and Hax-8 (δ 1.02) and between Hax-8 and H-10, indicating that they are all oriented to the opposite molecular face. These data were consistent with trans disposition of H-6 and H3-17. Furthermore, NOEs were observed between H-2 (δ 1.72) and H-6, between H-2 and H3-16 (δ 1.06) and between H3-17 and Heq-1 (δ 2.58), indicating that the C/D ring junction is cis. This cis junction was also supported by the fact that the chemical shift of methyl proton H3-17 was shifted upfield due to the anisotropic effect of the hydroxyphenyl group. Taken together, these findings suggested that 1 has a trans–transoid–trans–transoid–cis arrangement for the A–B–C–D ring system. Thus, the relative stereochemistry of 1 was presumed to be 2S*3R*6R*7R*10S*11S*.
Discussion
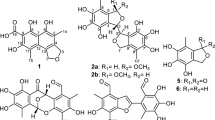
In the course of screening for microbial abrogators of bleomycin-induced G2 arrest in Jurkat cells, habiterpenol (1, Figure 1) was isolated from the culture broth of P. suffuscus 3787_5.1 In this study, the structure of 1 was elucidated to be a meroditerpenoid with a labdane-type diterpene connecting an indan moiety. A number of meroditerpenes having a benzofuro- or benzopyrano-ring moiety were isolated from natural sources, but five kinds of indan like moiety-containing meroterpenoids including 1 (Figure 1) have been reported so far. Sabry et al.2 isolated flabellinol and flabellinone from Stypopodium flabelliforme as potent inhibitors of the sodium channel. Abatis et al.3 isolated atomarianones A and B from Taonia atomari as cytotoxic agents in two lung cancer cell lines. Penicooke et al.4 isolated zonaquinone acetate from S. zonale as a cytotoxic agent in breast cancer and colon cancer cell lines. Interestingly, these meroditerpenoids were isolated from marine algae, while 1 was isolated from an actinomycete. The stereochemistries of these five meroditerpenoids differed as follows (Figure 1). Flabellinol and flabellinone have a unique cis–cisoid–cis–cisoid–trans A–B–C–D ring structure that induces rings A and C into chair conformations and ring B into a twist boat.2 Atomarianone A and zonaquinone acetate have an unprecedented trans–cisoid–cis–cisoid–trans A–B–C–D ring structure, while atomarianone B is the epimer of atomarianone A at C-7 constructing a trans–cisoid–trans–transoid–trans A–B–C–D ring structure.3 As elucidated in this study, 1 has a trans–transoid–trans–transoid–cis A–B–C–D ring structure that induces rings A, B and C into chair conformations; therefore, 1 has a unique ring structure among diterpens fused with an indane moiety in natural products. Further experiments, for example, X-ray crystallography or total synthesis, are needed to confirm the absolute stereochemistry of 1.
Methods
General
Various NMR spectra were obtained using an Agilent Technologies XL-400 (400 MHz) spectrometer (Agilent Technologies, Santa Clara, CA, USA). Electrospray ionization mass spectrometry (ESI-MS) was conducted on a JEOL JMS-T100LP spectrometer (Tokyo, Japan). UV–visible and IR spectra were measured with a Beckman DU640 spectrophotometer (Beckman Coulter, Inc., Fullerton, CA, USA) and a Horiba FT-210 Fourier transform infrared spectrometer (Horiba, Kyoto, Japan), respectively. Optical rotations was recorded on a JASCO model DIP-181 polarimeter (JASCO, Tokyo, Japan).
Producing actinomycete P. suffuscus 3787_5 and isolation of habiterpenol
The fermentation and purification procedures of 1 were described in the accompanying paper.1 The habiterpenol-producing actinomyces P. suffuscus 3787_5 was isolated from a soil sample collected on Ishigaki Island, Japan. Bioassay-guided fractionation of the culture broth of the actinomycete, including organic solvent extraction, ODS gel column chromatography and reversed phase (C4) HPLC, yielded 1.
References
Uchida, R. et alHabiterpenol, a novel abrogator of bleomycin-induced G2 arrest in Jurkat cells, produced by Phytohabitans suffuscus 3787_5. J. Antibiot. 67, 777–781 (2014).
Sabry, O. M. et al. Neurotoxic meroditerpenoids from the tropical marine brown alga Stypopodium flabelliforme. J. Nat. Prod. 68, 1022–1030 (2005).
Abatis, D. et al. Atomarianones A and B: two cytotoxic meroditerpenes from the brown alga Taonia atomaria. Tetrahedron Lett. 46, 8525–8529 (2005).
Penicooke, N. et al. Antiproliferative activity and absolute configuration of zonaquinone acetate from the Jamaican alga Stypopodium zonale. Phytochemistry 87, 96–101 (2013).
Acknowledgements
We express our thanks to Ms N Sato and Dr K Nagai, School of Pharmacy, Kitasato University, for measurements of NMR and mass spectra.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Uchida, R., Yokota, S. & Tomoda, H. Structure elucidation of meroterpenoid habiterpenol, a novel abrogator of bleomycin-induced G2 arrest in Jurkat cells, produced by Phytohabitans suffuscus 3787_5. J Antibiot 67, 783–786 (2014). https://doi.org/10.1038/ja.2014.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2014.71
This article is cited by
-
Species-specific secondary metabolism by actinomycetes of the genus Phytohabitans and discovery of new pyranonaphthoquinones and isatin derivatives
The Journal of Antibiotics (2023)
-
Habiterpenol, a novel abrogator of bleomycin-induced G2 arrest in Jurkat cells, produced by Phytohabitans suffuscus 3787_5
The Journal of Antibiotics (2014)