Abstract
Extracellular DNA is an adhesive component of staphylococcal biofilms. The aim of this study was to evaluate the antibiofilm activity of recombinant human DNase I (rhDNase) against Staphylococcus aureus and Staphylococcus epidermidis. Using a 96-well microtiter plate crystal-violet binding assay, we found that biofilm formation by S. aureus was efficiently inhibited by rhDNase at 1–4 μg l−1, and preformed S. aureus biofilms were efficiently detached in 2 min by rhDNase at 1 mg l−1. Pretreatment of S. aureus biofilms for 10 min with 10 mg l−1 rhDNase increased their sensitivity to biocide killing by 4–5 log units. rhDNase at 10 mg l−1 significantly inhibited biofilm formation by S. epidermidis in medium supplemented with sub-MICs of antibiotics. We also found that rhDNase significantly increased the survival of S. aureus-infected Caenorhabditis elegans nematodes treated with tobramycin compared with nematodes treated with tobramycin alone. We concluded that rhDNase exhibits potent antibiofilm and antimicrobial-sensitizing activities against S. aureus and S. epidermidis at clinically achievable concentrations. rhDNase, either alone or in combination with antimicrobial agents, may have applications in treating or preventing staphylococcal biofilm-related infections.
Similar content being viewed by others
Introduction
Adherent communities of bacteria known as biofilms have a role in the pathogenesis of many chronic infections.1 Biofilm bacteria synthesize an extracellular polymeric matrix that holds the cells together and attaches them to the surface.2 Extracellular DNA has been shown to be a component of the biofilm matrix produced by many bacteria.3 Functions ascribed to extracellular DNA include biofilm cohesion,4 cation chelation,5 biocide resistance6 and genetic exchange.7
Several studies have shown that exogenously added DNases can inhibit biofilm formation, detach preformed biofilms and sensitize biofilm bacteria to biocide killing.3 Most of these studies utilized laboratory grade DNase I isolated from bovine pancreas. Few studies have measured the antibiofilm activity of pharmaceutical grade recombinant human DNase I (rhDNase). The aim of the present study was to evaluate the effectiveness of rhDNase against biofilm-producing strains of Staphylococcus aureus and Staphylococcus epidermidis. In this report, we present evidence that rhDNase exhibits potent antibiofilm and antimicrobial-sensitizing activities against staphylococci in vitro and in vivo.
Materials and methods
Reagents
rhDNase (dornase alfa) was obtained from Genentech (South San Francisco, CA, USA). Dispersin B (DspB), a hydrolase that degrades poly-N-acetylglucosamine, was purified from a recombinant strain of Escherichia coli as previously described.8 Tigecycline was obtained from Pfizer (New York, NY, USA). Povidone iodine was purchased from Ricca (Arlington, TX, USA). Vancomycin, tobramycin, chlorhexidine gluconate and benzalkonium chloride were purchased from Sigma-Aldrich (St Louis, MO, USA).
Bacterial strains and growth conditions
The S. aureus strains used in this study were 8325,9 N315,10 SH1000,11 MZ100,12 Sa113,13 and KBI-11, a clinical isolate (provided by Dr George Zhanel of the University of Manitoba) that did not form significant biofilms in vitro. For S. epidermidis, the biofilm-forming reference strain RP62A (ATCC 35984) was employed. Strains were passaged weekly on blood agar and stored at 4 °C. For in vitro microtiter plate assays, biofilms were cultured in tryptic soy broth (TSB). All media were supplemented with 6 g l−1 of yeast extract and 8 g l−1 of glucose. All cultures were incubated at 37 °C.
Biofilm formation assay
Biofilms were cultured in 96-well polystyrene microtiter plates as previously described.6 Briefly, cells were diluted to 104–105 CFU ml−1 in TSB supplemented with antibiotics and enzymes at the indicated concentrations. Aliquots of cells (200 μl each) were transferred to the wells of a microtiter plate, and the plate was incubated at 37 °C for 18 h. Biofilms were washed with water and then stained for 1 min with 200 μl of Gram's crystal violet. Stained biofilms were rinsed with water and dried. The amount of biofilm biomass was quantified by destaining the biofilms for 10 min with 200 μl of 33% acetic acid and then measuring the absorbance of the crystal violet solution at 595 nm.
Biofilm killing assay
Biofilms were grown in 96-well microtiter plates as described above. Biofilms were rinsed with water and then treated with 180 μl of TSB supplemented with 10 mg l−1 rhDNase. Control wells were treated with 180 μl of TSB alone. After 10 min at 37 °C, a 20-μl volume of 4% chlorhexidine gluconate, 2% povidone iodine or 0.4% benzalkonium chloride was added to each well, and biofilms were incubated for 5 min at room temperature. Control wells received 20 μl of water. For biofilms pretreated with TSB alone, biofilms were rinsed four times with PBS to remove the biocide and then treated with 200 μl of 100 mg l−1 rhDNase (in 150 mM NaCl and 1 mM CaCl2) to dissolve the biofilm. After 10 min, cells were mixed and serial dilutions were plated on agar. For biofilms pretreated with rhDNase, cells were mixed and then quickly diluted 1:1000 in saline (50 μl of cells into 50 ml of saline). The cells were passed through an analytical test filter funnel (no. 145-2020; Nalgene, Rochester, NY, USA), and the filter was then rinsed with 250 ml of sterile water, aseptically removed from the filter unit, and placed on a blood agar plate. Colonies were enumerated after 24 h.
Caenorhabditis elegans liquid-medium killing assay
Infection of C. elegans nematodes with S. aureus was performed as previously described.14 Briefly, eggs of C. elegans DH26 (obtained from the Caenorhabditis Genetics Center) were hatched on lawns of E. coli OP50 on nematode growth medium agar. Worms were grown to L4 stage by incubation at 25 °C for 48 h. Worms were washed off the plate with M9 buffer, deposited onto lawns of S. aureus KBI-11 prepared according to Sifri et al.,15 and the plates were incubated at 25 °C for 17 h. The S. aureus-fed worms were washed from the plate with M9 buffer, allowed to settle for 5 min, rinsed with M9 buffer and 10–20 worms were transferred to the wells of a 96-well microtiter plate containing 100 μl of 80% M9 buffer, 20% TSB with or without 2.5 mg l−1 rhDNase and 1.25 mg l−1 tobramycin. Each treatment was assayed in triplicate. Plates were scored for live worms at the time of inoculation and every 24 h thereafter for 7 days using a dissecting microscope. Worms were considered dead if they appear straight and alive if they appeared S-shaped and were moving.
Statistics and reproducibility of results
All microtiter plate biofilm assays were performed in duplicate wells, which exhibited <10% variation in absorbance values. All assays were performed 2–3 times with similarly significant differences in absorbance values. The significance of differences between treatment groups was determined using the Student's t-test, with P-values of <0.05 considered significant. C. elegans infection assays were analyzed using survival curves generated by the Kaplan–Meier statistical method. The log rank test was used to compare survival differences for statistical significance using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA, version 4.0). P-values of <0.01 were considered significant.
Results
rhDNase inhibits S. aureus biofilm formation
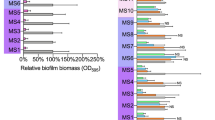
The ability of rhDNase to inhibit S. aureus biofilm formation was measured using a 96-well microtiter plate crystal-violet binding assay (Figure 1a). rhDNase at 4 μg l−1 efficiently inhibited biofilm formation by five biofilm-forming strains. The concentration of rhDNase required for a 90% reduction in absorbance values ranged from 0.125 μg l−1 for strain N315 to 4 μg l−1 for strain Sa113.
Inhibition (a) and detachment (b) of S. aureus biofilms by rhDNase in a 96-well microtiter plate crystal-violet binding assay. (a) The indicated strains were cultured in TSB supplemented with increasing concentrations of rhDNase. Absorbance at 595 nm is proportional to biofilm biomass. (b) Biofilms were cultured for 18 h in unsupplemented TSB, rinsed with water and then treated with rhDNase (1 mg l−1) for increasing amounts of time. For panels (a) and (b), values represent the means for duplicate wells. Error bars were omitted for clarity.
rhDNase detaches preformed S. aureus biofilms
The ability of rhDNase to detach preformed S. aureus biofilms was measured by culturing biofilms in 96-well microtiter plates and then treating the biofilms with 1 mg l−1 rhDNase for increasing amounts of time (Figure 1b). Under these conditions, rhDNase efficiently detached the biofilms produced by all five strains in 2–4 min.
rhDNase sensitizes S. aureus biofilms to biocide killing
We also measured the ability of rhDNase to sensitize preformed S. aureus SH1000 biofilms to killing by the topical biocides chlorhexidine gluconate, povidone iodine and benzalkonium chloride. As shown in Figure 2, biofilms exhibited a 1-log unit reduction in CFU per well values after a 5-min treatment with chlorhexidine gluconate at 0.4% or with povidone iodine at 0.2%. Biofilms were resistant to killing by benzalkonium chloride at 0.04%. Treatment of biofilms with 10 mg l−1 rhDNase for 10 min prior to the addition of the biocides resulted in a 4–5 log unit increase in bacterial killing. rhDNase alone did not exhibit significant antimicrobial activity.
Pretreatment of S. aureus biofilms with rhDNase renders them sensitive to killing by topical biocides. S. aureus SH1000 biofilms grown in 96-well microtiter plates were treated with the indicated agent for 5 min. In some wells, biofilms were pretreated with 10 mg l−1 rhDNase for 10 min prior to the addition of the agent. Values show mean numbers of surviving CFU per well, and error bars indicate range.
rhDNase enhances tobramycin killing of S. aureus in vivo
The ability of rhDNase to sensitize the S. aureus clinical strain KBI-11 to tobramycin killing was measured using a C. elegans in vivo infection model (Figure 3). Non-mammalian host organisms, including the soil nematode C. elegans, have previously been used to study host–pathogen interactions. Several human pathogens, including Gram-positive and Gram-negative bacteria, infect and kill C. elegans when they are supplied as a nutrient source.14, 15 In this experiment, nematodes were fed S. aureus cells for 17 h and then treated for 7 days with 1.25 mg l−1 tobramycin (2 × MIC) or 1.25 mg l−1 tobramycin plus 2.5 mg l−1 rhDNase. Tobramycin significantly increased the survival of nematodes after 1–7 days compared with the no-antibiotic control (P<0.01), and tobramycin plus rhDNase significantly increased the survival of nematodes after 1–7 days compared with tobramycin alone (P<0.01).
Killing of the nematode C. elegans by S. aureus in the presence of tobramycin and rhDNase. The graph shows Kaplan–Meier survival curves of uninfected nematodes, nematodes infected with S. aureus KBI-11 and nematodes infected with S. aureus KBI-11 and cultured in the presence of 2.5 mg l−1 rhDNase, 1.25 mg l−1 tobramycin, or 2.5 mg l−1 rhDNase plus 1.25 mg l−1 tobramycin.
rhDNase inhibits S. epidermidis biofilm formation
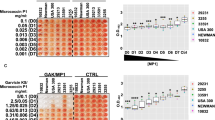
The ability of rhDNase to inhibit biofilm formation by S. epidermidis RP62A, a biofilm-forming reference strain, was measured using a 96-well microtiter plate assay (Figure 4). We also measured biofilm inhibition by rhDNase in combination with DspB, a glycoside hydrolase that degrades the major S. epidermidis biofilm matrix polysaccharide (poly-N-acetylglucosamine).6 Antibiotics were added at sub-MICs to induce biofilm formation.16, 17 rhDNase significantly inhibited biofilm formation in the absence or presence of antibiotics (P<0.05). rhDNase was more efficient than DspB at inhibiting S. epidermidis biofilm formation in the presence of a sub-MIC of tigecycline. DspB was more effective than rhDNase when tested alone and was comparable to rhDNase plus vancomycin. The combination of rhDNase and DspB inhibited biofilm formation more efficiently than either enzyme alone, both in the absence and presence of antibiotics.
Inhibition of S. epidermidis biofilm formation by rhDNase and DspB in 96-well microtiter plates. S. epidermidis strain RP62A was cultured in the presence of 10 mg l−1 rhDNase, 10 mg l−1 DspB, or a combination of both enzymes at 10 mg l−1 each. Some wells were further supplemented with 1.5 mg l−1 vancomycin (2/3 × MIC), 0.02 mg l−1 tigecycline (1/4 × MIC). After 16 h, biofilms were rinsed with water and quantified by crystal violet staining as described in the legend to Figure 1. Values show mean absorbance values for duplicate wells, and error bars indicate range.
Discussion
Extracellular DNA has been shown to be an adhesive component of the biofilm matrix synthesized by many different bacteria.3 Among the staphylococci, extracellular DNA has been shown to be the major matrix component in S. aureus biofilms, but only a minor matrix component in S. epidermidis biofilms.6
Previous studies showed that various DNases, including bovine DNase I, micrococcal nuclease, restriction endonucleases and human DNase1L2, are capable of inhibiting biofilm formation or detaching preformed biofilms produced by various bacterial species.6, 18 Few studies have measured the antibiofilm activity of rhDNase. Hall-Stoodley et al.19 showed that a 15-min treatment with rhDNase at 1 mg l−1 caused a 10–73% decrease in the biomass of Streptococcus pneumoniae biofilms cultured in microtiter plates as determined by COMSTAT analysis. However, rhDNase concentrations of 1 g l−1 were required to cause a 67–95% decrease in biofilm biomass. Parks et al.20 showed that a 10-min treatment with rhDNase at 33 mg l−1 caused a 75% reduction in the biomass of neutrophil-induced 24-h-old Pseudomonas aeruginosa biofilms cultured in microtiter plates as determined by crystal violet staining. However, biofilms grown for 48 h were reduced by only 42%.
In the present study, we measured the antibiofilm activity of rhDNase against S. aureus and S. epidermidis. We found that rhDNase at 0.125–4 μg l−1 efficiently inhibited biofilm formation by S. aureus (Figure 1a). Previous studies showed that under conditions identical to those used in the present study, bovine DNase I at 100 mg l−1 resulted in 40–50% inhibition of biofilm formation by S. aureus strains SH1000 and MRSA252.6 Thus, rhDNase may be a more potent inhibitor of S. aureus biofilm formation than bovine DNase I. We also found that rhDNase rapidly and efficiently detached S. aureus biofilms (Figure 1b) and sensitized them to biocide killing (Figure 2) at clinically achievable concentrations. Furthermore, rhDNase significantly increased the survival of S. aureus-fed C. elegans nematodes exposed to tobramycin (Figure 3), even with a S. aureus strain that did not form biofilms in vitro. These findings underscore the importance of extracellular DNA in S. aureus biofilm cohesion and antimicrobial resistance.6
In the present study, we found that rhDNase at 10 mg l−1 significantly inhibited biofilm formation by S. epidermidis strain RP62A (Figure 4), but the amount of biofilm inhibition was only 25%. These findings are consistent with those of previous studies showing that bovine DNase I concentrations of 2–4 g l−1 were required to efficiently inhibit biofilm formation by S. epidermidis strain 1457.21 In the present study, rhDNase was not as effective as DspB at inhibiting S. epidermidis biofilm formation in the absence of antibiotics, but was equally or more effective than DspB at inhibiting biofilm formation in the presence of sub-MICs of tigecycline or vancomycin. These findings suggest that S. epidermidis may produce extracellular DNA-dependent biofilms in the response to sub-MICs of antibiotics.16, 17 Biofilm inhibition was most efficient in the presence of both rhDNase and DspB, supporting the hypothesis that both extracellular DNA and poly-N-acetylglucosamine surface polysaccharide contribute to S. epidermidis biofilm cohesion.6
In summary, rhDNase efficiently inhibits S. aureus and S. epidermidis biofilm formation, and disperses preformed S. aureus biofilms in vitro. In addition, rhDNase sensitizes S. aureus biofilms to biocide killing in vitro and S. aureus cells to antibiotic killing in vivo. Furthermore, the combination of rhDNase and DspB is more efficient than either enzyme alone in inhibiting S. epidermidis biofilm formation in the absence or presence of antibiotics in vitro. Thus, rhDNase, either alone or in combination with antimicrobial agents, may have potential applications in developing products for the prevention and treatment of staphylococcal biofilm infections, such as those associated with cystic fibrosis, chronic wounds and medical implants.
One of the main concerns with enzyme-based antibiofilm therapy is that the dispersal of cells from the biofilm colony may increase the risk of bloodstream infections and localized infections at distant sites. Enzyme-based therapies may need to be performed in combination with antimicrobial agents, or their utility may be limited to preventive rather than treatment modalities. In addition, rhDNase may interfere with the structure and function of neutrophil extracellular traps. Another drawback to the use of enzymes is their high cost compared with conventional chemical disinfectants and antibiotics. More studies will be needed to evaluate the potential clinical utility of rhDNase as an anti-infective therapeutic.
References
Donlan, R. M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. 8, 881–890 (2002).
Flemming, H- C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Kaplan, J. B. Therapeutic potential of biofilm-dispersing enzymes. Int. J. Artif. Organs 32, 545–554 (2009).
Whitchurch, C. B. et al. Extracellular DNA required for bacterial biofilm formation. Science 295, 1487 (2002).
Mulcahy, H., Charron-Mazenod, L. & Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLOS Pathog. 4, e1000213 (2009).
Izano, E. A. et al. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl. Environ. Microbiol. 74, 470–476 (2008).
Molin, S. & Tolker-Nielsen, T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr. Opin. Biotechnol. 14, 255–261 (2003).
Yakandawala, N. et al. Enhanced expression of engineered ACA-less β-1,6-N-acetylglucosaminidase (dispersin B) in Escherichia coli. J. Ind. Microbiol. Biotechnol. 36, 1297–1305 (2009).
Novick, R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33, 155–166 (1967).
Kuroda, M. et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357, 1225–1240 (2001).
Horsburgh, M. J. et al. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184, 5457–5467 (2002).
Shanks, R. M. Q. et al. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73, 4596–4606 (2005).
Iordanescu, S. & Surdeanu, M. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96, 277–281 (1976).
Moy, T. I. et al. Identification of novel antimicrobials using a live-animal infection model. Proc. Natl Acad. Sci. USA 103, 10414–10419 (2006).
Sifri, C. D. et al. Caenorhabditis elegans as a model host for Staphylococcus aureus pathogenesis. Infect. Immun. 71, 2208–2217 (2003).
Cargill, J. S. & Upton, M. Low concentrations of vancomycin stimulate biofilm formation in some clinical isolates of Staphylococcus epidermidis. J. Clin. Pathol. 62, 1112–1116 (2009).
Rachid, S. et al. Effects of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob. Agents Chemother. 44, 3357–3363 (2000).
Eckhart, L. et al. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 156, 1342–1345 (2007).
Hall-Stoodley, L. et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 8, 173 (2008).
Parks, Q. M. et al. Neutrophil enhancement of Pseudomonas aeruginosa biofilm development: human F-actin and DNA as targets for therapy. J. Med. Microbiol. 58, 492–502 (2009).
Qin, Z. et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 153, 2083–2092 (2007).
Acknowledgements
We thank Pierre Hardouin and Thierry Grard of the Université du Littoral-Côte d’Opale (Boulogne-sur-mer, France), in whose laboratory some of the work was performed. We also thank George Zhanel of the University of Manitoba for providing S. aureus strain KBI-11. This study was funded by grants from the US Department of State Fulbright Commission, the Nord-Pas de Calais Regional Council (France), the National Institute of Allergy and Infectious Diseases (award no. AI82392) and Genentech, Inc. (to JBK); and by Manitoba Health Research Council operating grant 309342 (to STC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JBK receives research funding from Genentech, Inc. This company may develop anti-infective applications based on rhDNase. JBK owns equity in, receives royalty payments from and serves on the advisory board of Kane Biotech, Inc. KL and SM own equity in and are employed by Kane Biotech, Inc. This company is developing anti-infective applications based on DspB. Other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kaplan, J., LoVetri, K., Cardona, S. et al. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J Antibiot 65, 73–77 (2012). https://doi.org/10.1038/ja.2011.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2011.113
Keywords
This article is cited by
-
Microbubble cavitation restores Staphylococcus aureus antibiotic susceptibility in vitro and in a septic arthritis model
Communications Biology (2023)
-
Using next generation antimicrobials to target the mechanisms of infection
npj Antimicrobials and Resistance (2023)
-
Antimicrobial Peptides Against Microbial Biofilms: Efficacy, Challenges, and Future Prospect
International Journal of Peptide Research and Therapeutics (2023)