Abstract
A new bafilomycin-type macrolide, named hygrobafilomycin (6), was isolated by a bioassay-guided selection and fractionation from a terrestrial actinomycete, Streptomyces varsoviensis, along with three known derivatives, bafilomycin D (3), C1 (4) and C2 (5). The structure of hygrobafilomycin was fully established by MS and NMR analyses, revealing a hygrolidin–bafilomycin hybrid with an unusual monoalkylmaleic anhydride moiety. Hygrobafilomycin (6) shows strong antifungal, antiproliferative and cytotoxic activities. In a panel of 40 tumor cell lines, compound 6 shows high cytotoxic potency (mean IC50=5.3 n).
Similar content being viewed by others
Introduction
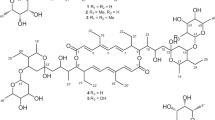
Hygrolidin (1) and bafilomycin A1 (2) (Figure 1) are the prototypes of a group of 16-membered macrolides known as the hygrolides.1 Natural hygrolidin and bafilomycin derivatives differ in the substitution pattern of the polyketide backbone (C-2 and tetrahydropyran ring), as well as optional side chains. These well-known actinomyces metabolites are pharmacologically important, because of their biological activities including antiparasitic, antitumor and immunosuppressant,2, 3, 4, 5 together with bactericidal, fungicidal, insecticidal and herbicidal effects.6, 7, 8 Bafilomycin A1 (2) is a highly potent and specific inhibitor of vacuolar ATPase enzymes,9, 10 and has a role as carrier type K+ ionophore,11 which has led to a considerable interest in these metabolites. However, the practical use of these macrolides has been limited because of fatal toxicity,12 thus propelling semi-synthetic and biosynthetic engineering programs with the aim to create novel derivatives with better efficacy.13, 14 In this study, we report the isolation and structure characterization of a cytotoxic antifungal bafilomycin–hygrolidin hybrid, named hygrobafilomycin (6), featuring an unprecedented monoalkylmaleic anhydride moiety, and its biological evaluation using a panel of tumor cell lines.
Results and Discussion
The organic extracts of cultures of a Streptomyces varsoviensis strain (HKI0553) showed strong cytotoxic activities in a panel of six tumor cell lines differing in chemosensitivity toward standard chemotherapeutic agents (gastric cancer GXF 251L, non-small cell lung cancer LXF 529L, breast cancer MAXF 401NL, melanoma MEXF 462NL, renal cancer RXF 486L and uterus cancer UXF 1138L). LC-DAD and MS analysis of the metabolic profile revealed several compounds with UV spectra characteristic for bafilomycins, including one novel congener as judged from the molecular ion of m/z 757 (M+H)+. Mycelium harvested from an up-scaled fermentation (200 l) was extracted with MeOH and the dried extract was fractionated by flash chromatography using a CHCl3/MeOH gradient. The CHCl3 fraction showing strong antifungal and cytotoxic activities was subjected to successive chromatography using open columns with silica gel and Sephadex LH-20. The final purification was achieved by preparative RP-HPLC, yielding three known bafilomycins, D (3, 10 mg), C1 (4, 13.7 mg) and C2 (5, 25 mg) together with the new derivative 6 (5 mg) (Figure 2). The structures of compounds 3 to 6 were solved by MS and NMR spectroscopy and confirmed by comparison with literature data for the known bafilomycins.6, 8
Structure elucidation
Compound 6 was obtained as a white amorphous powder. The molecular formula C42H60O12; was determined by HRESI-MS m/z 757.3999 (M+H)+ and predicts 13 degrees of unsaturation. The UV and IR spectra are characteristic for bafilomycins and indicate the presence of OH groups and conjugated carbonyl groups. The 13C NMR spectrum of 6 shows 41 signals for 42 carbons (two of which had the same chemical shift δ 135.1). In conjunction with the DEPT spectrum, the presence of 1 methoxy group at δ 55.9, 10 methyl groups, 2 methylene groups, 6 methine carbon atoms, 8 ethylenic carbon atoms, 6 oxymethine carbon atoms, 4 ethylenic quaternary carbon atoms, 1 quaternary sp3carbon atom at δ 104.2 and 4 carbonyl groups of esters at δ 166.2, 167.8, 168.0 and 172.8, was concluded. Although the NMR data for 6 (Table 1) proved to be very similar to the spectroscopic data of bafilomycin A1,15 they show significant differences. First, compound 6 has only one methoxy group, while all known bafilomycins have at least two methoxy groups. The number of methyl groups, 10 for compound 6 and 9 for most bafilomycins suggest that 1 methoxy group, likely at C-2, is replaced by a methyl group. This was confirmed by the 13C NMR chemical shifts of carbons C-2 (δ 123.0) and C-3 (δ 147.9) (similar to 116) and the HMBC correlations of 3-H with C-1, C-26 and 2-CH3 (Figure 3). Second, compared with 2,15 compound 6 possesses three additional ethylenic carbons, one quaternary sp2carbon and three ester carbonyl groups. The HMBC correlations of 21-H with C-1′and 3′-H (or 2′-H) with C-1′ support the presence of an α,β-unsaturated ester attached to the tetrahydropyran ring. The E configuration of the olefin was deduced from the coupling constant of the two olefinic protons (J=11.3 Hz), which appear at the same chemical shift δ 6.77. The presence of an alkylmaleic anhydride moiety was also supported by HMBC correlations (Figure 3). The relative configuration of 6 was deduced by comparison of the coupling constants with 115 and 2.16 For all compounds, the low coupling constant of 7-H (<6 Hz) is indicative for the cis axial orientation between 6-H, 7-H and 8-H (Figure 3). These assignments are also supported by NOESY correlations between 6-H/8-H, 14-H/30-H, 18-H/30-H, 21-H/23-H and 21-H/32-H, and are in accordance with the previously reported relative configuration of the known co-metabolites bafilomycins (3, 4 and 5).
In sum, the configuration of compound 6 and the substitution pattern of the tetrahydropyran ring are in full accord with that of the known bafilomycins. Yet, as the macrolide portion is identical to hygrolidin (1), compound 6 represents a novel bafilomycin–hygrolidin hybrid named hygrobafilomycin (6), which is also unique because of its rare monoalkylmaleic anhydride moiety.
Alkylmaleic anhydride finds applications in nearly every field of both laboratory and industrial chemistry, primarily as a synthetic building block in organic chemistry.17, 18 In stark contrast, the occurrence of alkylmaleic anhydride units in natural products is scarce.19 Although several natural products containing a dialkylmaleic anhydride unit are known, only meliacinanhydride and C21 norsesterterpene acid represent natural products with a monoalkylmaleic anhydride.20, 21
Biosynthetic considerations
The structure of 6 is intriguing from a biosynthetic point of view, as the polyketide core shares structural features of both the bafilomycins and the hygrolidins, and carries an unprecedented side chain. Unfortunately, isotope-labeling experiments were hampered because of the minute amounts of 6 produced (25 μg l−1). Thus, one can only make an educated guess on the basis of the models for the bafilomycin and tautomycin biosynthetic pathways. Undoubtedly, the formation of the polyketide-derived macrolide of 6 would be in line with the biosynthesis of bafilomycin A1 (2),22 albeit the final methoxymalonyl extender unit23 would be replaced by a methylmalonyl building block. Owing to the co-occurrence of 6 with three bafilomycin derivatives with C-2 methoxy groups (3–5), it seems that the acyl transferase domain of the terminal polyketide synthase module has some degree of flexibility toward the methyl and methoxy malonyl extenders.24
As for the alkylmaleic anhydride substituent, one may deduce a biosynthetic scheme from the tautomycin pathway.25 Osada and colleagues25 suggested that the dialkylmaleic anhydride moiety is biosynthesized through an aldol condensation of methylmalonyl-CoA and α-ketoglutarate derived from the Krebs cycle. In analogy, a plausible route toward the monoalkylmaleic anhydride side chain of 6 would involve an aldol condensation of acetyl-CoA with the C-5 unit derived from α-keto glutarate, followed by dehydration. Subsequent hydroxylation and dehydration could yield the double bond at position C-2′/C-3′.26 The final step would be the transfer of the monoalkylmaleic anhydride side chain onto the polyketide backbone as has been shown for tautomycin biosynthesis.
Biological activity
Compounds 2, 3, 4, 5 and 6 were subjected to cytotoxic testing in a panel consisting of 40 different human tumor cell lines, reflecting 15 tumor types (bladder, colon, stomach, head and neck, liver, lung, breast, melanoma, ovary, pancreas, prostate, pleuramesothelioma, kidney, sarcoma and uterus). Overall, compounds 2, 4, 5 and 6 show very strong cytotoxic activities with mean IC50 values of 3.6, 1.7, 5.0 and 5.3 nM, respectively (for individual IC50 values see supplementary information). Data are consistent with previous reports regarding bafilomycin A1 (2), tested against Capan-1 pancreatic cancer cells and showing antitumor effects in vitro (IC50=5 nM) and growth inhibition of the xenograft in vivo.27 The compounds show selective cytotoxicity with above average activity in 12.5–17.5% of the cell lines as tested (Table 2). The lower cytotoxic activity of compound 3 (mean IC50 86.3 nM) points to a crucial role of the tetrahydropyran ring system, as this was found for the vacuolar ATPase inhibition effect of bafilomycin derivatives.13 A particular importance of the maleic anhydride moiety, as found in other cases, could not be connoted.19 As the compounds show only low cell line selectivities, a basic physiological function as cellular target seems plausible. However, comparison of the antitumor analysis with 94 reference compounds by the COMPARE algorithm did not show any significant correlation (rho<0.5 for all compounds), and thus the mechanism of action remains unclear.28 Besides the detailed cytotoxicity screening, the compounds were also subjected to a basic antibacterial and antifungal testing (see Supplementary Information). Again, we found that compounds 4, 5 and 6 have a much more pronounced inhibitory activity than compound 3. In this study, it should be noted that the antifungal activity of all compounds is by far more pronounced than the antibacterial effects, and gram-negative bacteria seem to be completely insensitive toward 3–6, as has been reported for bafilomycins A–C.6
In summary, we have isolated a new bafilomycin–hygrolidin derivative, hygrobafilomycin (6) from the mycelium of a S. varsoviensis culture. The structure of 6 was fully established by MS and NMR techniques and revealed a bafilomycin–hygrolidin hybrid scaffold featuring a rare monoalkylmaleic anhydride unit, which is fully unprecedented for bafilomycins. This moiety is intriguing from a biosynthetic point of view and may account, at least in part, for the biological activities observed in our assays. Hygrobafilomycin (6) shows pronounced cytotoxic activity against several cell lines derived from solid human tumors, as well as pronounced antifungal activity. Owing to its high cytotoxic potency (mean IC50=5.3 nM), this unusual hybrid molecule represents an important addition to the family of antitumoral hygrolides.
Experimental section
General experimental procedures
NMR spectra were recorded on Bruker DPX-300 and a Bruker Avance III (Bruker Biospin GmbH, Karlsruhe, Germany) 500 MHz spectrometers; chemical shifts are given in δ values (p.p.m.). IR spectra were recorded on a Bruker FT-IR (IFS 55) spectrometer. UV spectra were recorded on a Cary 1 Bio UV-visible spectrophotometer (Varian GmbH, Darmstadt, Germany). LC-MS measurements were recorded on Agilent 1100 Series (Hewlett-Packard GmbH, Waldbronn, Germany) LC/MSD Trap. HRESI-MS were recorded on a Finnigan TSQ quantum ultra (Thermo Fisher Scientific GmbH, Dreieich, Germany) mass spectrometer. Optical rotation was measured using a 0.5 dm cuvette with a Jasco P-1020 polarimeter (Jasco, Gotha, Germany) at 25 °C. Open column chromatography was performed on silica gel 60 (Merck KGaA, Darmstadt, Germany, 0.04–0.063 mm, 230–400 mesh ASTM) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden). TLC: silica gel plates (silica gel 60F254 on aluminum foil or glass, Merck); spots were visualized by spraying with anisaldehyde/sulfuric acid followed by heating. Analytical HPLC was conducted on a Shimadzu SPD-M10A (Shimadzu Corporation, Kyoto, Japan) with diode array detector system, using a Phenomenex fusion-RP column (Phenomenex, Aschaffenburg, Germany) (4 μm, 250 × 4.6 mm) with MeCN per 0.1% TFA-H2O as eluent (flow rate 1 ml min−1; 25–75% in 8 min, 75–95% in 22 min and 95–100% MeCN in 5 min) and UV detection at 254 nm. Preparative HPLC was performed on a Shimadzu LC-8A with a diode array detector system, using a Phenomenex column. All solvents used were spectral grade or distilled before use.
Taxonomy of the producing strain
Phenotypic identification as S. varsoviensis was confirmed by 16S rDNA analysis (AMODIA Bioservice GmbH, Braunschweig, Germany) and sequence comparison with GenBank data (National Center for Biotechnology Information; NIH, USA) by 99.0% similarity.
Culture material and fermentation
Storage of the pure strain was carried out in 50% glycerol at –80 °C. The strain was grown in 20 ml of medium 79 (dextrose 10 g, peptanbacto 10 g, casamino acids 1.0 g, yeast extract 2.0 g and NaCl 6.0 g in 1.0 l distilled water) for 6 days at 28 °C. Two times 6.0 ml of this culture were used to inoculate two 500-ml Erlenmeyer flasks containing 100 ml of medium 1 (composed of soy meal 20 g and mannitol 20 g in 1.0 l distilled water, pH=7.5 before sterilization). The cultures were shaken on a rotary shaker (180 r.p.m.) at 28 °C for 5 days. Each of these 100 ml cultures were used to inoculate 1.0 l of medium 1. This 2.0 l culture was used to inoculate 20 l of medium 1; after 4 days, this was further used as seed culture for 175 l of medium 1 in a 300 l fermentor vessel at pH 7.0−7.2. The fermentation was carried out for 6 days with aeration at 30 l min−1 and stirring at 200 r.p.m.
Extraction and isolation
From a large-scale fermentation (200 l), mycelium was separated from the culture filtrate by filtration and extracted with methanol. The dried extract (200 g) was fixed on silica gel (220 g) and fractionated through flash chromatography in three fractions: CHCl3 (40 g), CHCl3/MeOH 1:1 (60 g) and MeOH (75 g); with 25 g of fat. The CHCl3 fraction showed strong antifungal and cytotoxic activities and was chosen for further purification. This fraction was successively subjected to open column chromatography using silica gel (gradient of CHCl3/MeOH) and Sephadex LH-20 (eluent MeOH) respectively. Final purification of the bafilomycins derivatives was achieved by preparative RP-HPLC: Phenomenox fusion-RP 250 × 21 mm, flow rate 12 ml min−1, gradient MeOH-H2O 25–85% in 8 min, 85–95% in 22 min and 95–100% MeOH in 5 min, UV detection at 254 nm. This afforded four compounds, 3 (10 mg), 4 (13.7 mg), 5 (25 mg) and 6 (5 mg).
Compound 6
White amorphous powder; [α]21D +33.0 (c 0.14, MeOH); UV λmax (MeOH) nm: 282, 246 and 203; IR (KBr) cm−1: 3457, 2965, 2359, 2325, 1721, 1677, 1648 and 1256; 1H NMR (300 MHz, CD3OD) and 13C NMR (75 MHz, CD3OD) see Table 1; HRESI-MS m/z 757.3999 (M+H)+, calcd 756.3912 for C42H60O12.
Monolayer assay
A modified propidium iodide assay was used to determine the cytotoxic activity of the compounds against human tumor cell lines. The test procedure has been described elsewhere.29 Cell lines tested were derived from patient tumors engrafted as a subcutaneously growing tumor in NMRI nu/nu mice, or obtained from the American Type Culture Collection, Rockville, MD, USA, National Cancer Institute, Bethesda, MD, USA, or Deutsche Sammlung von Mikroorganismen und Zellkulturen, Braunschweig, Germany. Briefly, human tumor cells lines were grown at 37 °C in a humidified atmosphere (95% air, 5% CO2) in monolayer cultures in RPMI 1640 medium supplemented with 10% fetal calf serum and phenol red (PAA, Cölbe, Gemany). Cells were trypsinized and maintained weekly. Cells were harvested from exponentially growing cultures by trypsination, counted and plated in 96-well flat-bottomed micro plates (140 μl cell suspension, 5 × 103–10 × 103 cells per well). After a 24 h recovery to allow cells to resume exponential growth, 10 μl of culture medium (six control wells per plate) or medium containing the test drug were added to the wells. Each drug concentration was plated in triplicate. After 4 days of incubation, the culture medium was replaced by fresh medium containing 6 μg ml−1 of propidium iodide. Microplates were then kept at –18 °C for 24 h, to give a total cell kill. After thawing of the plates, fluorescence was measured using the Cytofluor 4000 microplate reader (MTX Lab Systems Inc., McLean, VA, USA) (excitation 530 nm, emission 620 nm). The amount of viable cells was proportional to the fluorescence intensity.
For the COMPARE analysis, antitumor selectivity patterns were compared by ranking and correlating individual IC-values of a test compound with those IC-values of standard agents in the same tumor models.28 Similarities in antitumor selectivity of the test compound to those of the standard drugs were expressed quantitatively by the Spearman rank correlation coefficient rho (ρ). Compounds that have a high correlation coefficient have generally been found to have similar mechanisms of action. Spearman correlations with ρ⩾0.5 were considered as relevant. A high correlation (ρ⩾0.6) to a specific standard agent indicated a similar mechanism of action (COMPARE-positive). Low correlations to all standard agents indicated a mechanism of action that was not represented by the standard agent database, or could even represent an unknown mechanism of action (COMPARE-negative).
References
Seto, H., Tajima, I., Akao, H., Furihata, K. & Otake, N. The isolation and structures of hygrolidin amide and defumarylhygrolidin. J. Antibiot. 37, 610–613 (1984).
Goetz, M. A. et al. L-155,175—a new antiparasitic macrolide fermentation, isolation and structure. J. Antibiot. 38, 161–168 (1985).
Wilton, J. H., Hokanson, G. C. & French, J. C. Pd-118,576—a new antitumor macrolide antibiotic. J. Antibiot. 38, 1449–1452 (1985).
Kawada, M. et al. Hygrolidin induces p21 expression and abrogates cell cycle progression at G1 and S phases. Biochem. Biophys. Res. Commun. 298, 178–183 (2002).
Vanek, Z., Mateju, J. & Curdova, E. Immunomodulators isolated from microorganisms. Folia Microbiol. 36, 99–111 (1991).
Werner, G., Hagenmaier, H., Drautz, H., Baumgartner, A. & Zahner, H. Metabolic products of microorganisms 0.224. bafilomycins, a new group of macrolide antibiotics production, isolation, chemical-structure and biological-activity. J. Antibiot. 37, 110–117 (1984).
Kretschmer, A., Dorgerloh, M., Deeg, M. & Hagenmaier, H. The structures of novel insecticidal macrolides—bafilomycin-D and bafilomycin-E, and oxohygrolidin. Agric. Biol. Chem. 49, 2509–2511 (1985).
Kim, S.- D., Ryoo, I.- J., Kim, C.- J., Uramoto, M. & Yoo, I.- D. The structure determination of a herbicidal compound, 3D5. J. Microbiol. Biotech. 3, 51–56 (1993).
Drose, S. et al. Inhibitory effect of modified bafilomycins and concanamycins on P-type and V-type adenosine-triphosphatases. Biochemistry 32, 3902–3906 (1993).
Gagliardi, S., Rees, M. & Farina, C. Chemistry and structure activity relationships of bafilomycin A(1), a potent and selective inhibitor of the vacuolar H+-ATPase. Curr. Med. Chem. 6, 1197–1212 (1999).
Teplova, V. V., Tonshin, A. A., Grigoriev, P. A., Saris, N. E. L. & Salkinoja-Salonen, M. S. Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J. Bioenerg. Biomembr. 39, 321–329 (2007).
Bowman, E. J., Siebers, A. & Altendorf, K. Bafilomycins—a class of inhibitors of membrane Atpases from microorganisms, animal-cells, and plant-cells. Proc. Natl Acad. Sci. USA 85, 7972–7976 (1988).
Gagliardi, S. et al. Synthesis and structure-activity relationships of bafilomycin A(1) derivatives as inhibitors of vacuolar H+-ATPase. J. Med. Chem. 41, 1883–1893 (1998).
Bowman, E. J. & Bowman, B. J. V-ATPases as drug targets. J. Bioenerg. Biomembr. 37, 431–435 (2005).
Baker, G. H., Brown, P. J., Dorgan, R. J. J. & Everett, J. R. The conformational-analysis of bafilomycin-A1. J. Chem. Soc., Perkin Trans. 2, 1073–1079 (1989).
Seto, H., Akao, H., Furihata, K. & Otake, N. The structure of a new antibiotic, hygrolidin. Tetrahedron Lett. 23, 2667–2670 (1982).
Adlington, R. M., Baldwin, J. E., Cox, R. J. & Pritchard, G. J. A rapid entry to natural and unnatural disubstituted maleic anhydrides. Synlett. 820–822 (2002).
Kar, A. & Argade, N. P. A facile access to natural and unnatural dialkylsubstituted maleic anhydrides. Tetrahedron 59, 2991–2998 (2003).
Chen, X. L., Zheng, Y. G. & Shen, Y. C. Natural products with maleic anhydride structure: Nonadrides, tautomycin, chaetomellic anhydride, and other compounds. Chem. Rev. 107, 1777–1830 (2007).
Siddiqui, B. S., Afshan, F., Gulzar, T. & Hanif, M. Tetracyclic triterpenoids from the leaves of Azadirachta indica. Phytochemistry 65, 2363–2367 (2004).
Wang, N. et al. Sesterterpenoids from the sponge Sarcotragus sp. J. Nat. Prod. 71, 551–557 (2008).
Schuhmann, T. & Grond, S. Biosynthetic investigations of the v-type ATPase inhibitors bafilomycin A(1), B-1 and concanamycin A. J. Antibiot. 57, 655–661 (2004).
Schuhmann, T., Vollmar, D. & Grond, S. Biosynthetic origin of the methoxyl extender unit in bafilomycin and concanamycin using stereospecifically labeled precursors. J. Antibiot. 60, 52–60 (2007).
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Li, W. L., Ju, J. H., Rajski, S. R., Osada, H. & Shen, B. Characterization of the tautomycin biosynthetic gene cluster from Streptomyces spiroverticillatus unveiling new insights into dialkylmaleic anhydride and polyketide biosynthesis. J. Biol. Chem. 283, 28607–28617 (2008).
Ubukata, M., Cheng, X. C., Uzawa, J. & Isono, K. Biosynthesis of the dialkylmaleic anhydride-containing antibiotics, tautomycin and tautomycetin. J. Chem. Soc., Perkin Trans. 1 2399–2404 (1995).
Ohta, T. et al. Bafilomycin A(1) induces apoptosis in the human pancreatic cancer cell line Capan-1. J. Pathol. 185, 324–330 (1998).
Fiebig, H. H. et al. High throughput screen for anticancer compounds based on human tumor cell lines and bioinformatics. Proc. Am. Assoc. Cancer Res. 46, 3967 (2005).
Dengler, W. A., Schulte, J., Berger, D. P., Mertelsmann, R. & Fiebig, H. H. Development of a propidium Iodide fluorescence assay for proliferation and cytotoxicity assays. Anti-Cancer Drugs 6, 522–532 (1995).
Acknowledgements
Financial support by the BMBF (FKZ 0315153) is gratefully acknowledged. We thank Heike Heinecke and Ulrike Valentin for technical assistance, Franziska Rhein for NMR measurements, Andrea Perner for MS measurements, Jutta Fehr and Roger Vollmer for cytotoxicity assays, Maria-Gabriele Schwinger and Christiane Weigel for antimicrobial assays.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website
Supplementary information
Rights and permissions
About this article
Cite this article
Tchize Ndejouong, B., Sattler, I., Maier, A. et al. Hygrobafilomycin, a cytotoxic and antifungal macrolide bearing a unique monoalkylmaleic anhydride moiety, from Streptomyces varsoviensis. J Antibiot 63, 359–363 (2010). https://doi.org/10.1038/ja.2010.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.52
Keywords
This article is cited by
-
Biosynthesis of Methoxymalonyl-acyl Carrier Protein (ACP) as an Extender Unit for Bafilomycin Polyketide in Streptomyces griseus DSM 2608
Biotechnology and Bioprocess Engineering (2018)
-
Biosynthesis of 2-amino-3-hydroxycyclopent-2-enone moiety of bafilomycin in Kitasatospora cheerisanensis KCTC2395
Journal of Microbiology (2018)
-
Bafilomycin L, a new inhibitor of cholesteryl ester synthesis in mammalian cells, produced by marine-derived Streptomyces sp. OPMA00072
The Journal of Antibiotics (2015)
-
Antifungal Macrolides from Streptomyces cavourensis YY01-17
Chemistry of Natural Compounds (2013)
-
Antifungalmycin, an antifungal macrolide from Streptomyces padanus 702
Natural Products and Bioprospecting (2012)