Abstract
Prostate stroma can regulate the growth and metastasis of prostate cancer through the tumor–stromal cell interactions. Thus, small molecules that modulate the tumor–stromal cell interactions will have a chance to become new antitumor drugs. In the course of our screening of the modulators, we isolated three new natural compounds, NBRI16716A (1), NBRI16716B (2) and NBRI16716C (3), from the fermentation broth of Perisporiopsis melioloides Mer-f16716, although compound 2 was already reported as a chemical degradation product of isotriornicin. Compounds 1 and 2 inhibited the growth of human prostate cancer DU-145 cells in the coculture with human prostate stromal cells (PrSCs) more strongly than that of DU-145 cells alone. Furthermore, both compounds showed antitumor effect against xenograft models of DU-145 cells and PrSCs in vivo.
Similar content being viewed by others
Introduction
Growing evidence supports the idea that the growth and metastasis of prostate cancer are regulated by prostate stroma.1, 2 We focused on such tumor–stromal cell interactions of prostate cancer and reported that prostate stromal cells (PrSCs) promote the growth of human prostate cancer cells through secretion of insulin-like growth factor-I.3, 4 Because small molecules that modulate the tumor–stromal cell interactions possibly show potent antitumor effect, we developed the in vitro coculture system of human prostate cancer cells and PrSCs, in which the growth of prostate cancer cells is increased by the coculture with PrSCs.3, 5 Using the assay method as a screening system for antitumor compounds, we have been finding several compounds that exert antitumor effects through modulation of the tumor–stromal cell interactions.6, 7 We have recently reported that leucinostatin A, a fungus natural compound, showed antitumor effect against xenograft models of human prostate cancer cells in vivo through the downregulation of insulin-like growth factor-I secretion from PrSCs.8 By continuing the screening system, we have found new natural compounds from a fungal strain Perisporiopsis melioloides Mer-f16716. In this study we describe the isolation, structure determination and biological activity of NBRI16716A (1), NBRI16716B (2) and NBRI16716C (3).
Results
Isolation procedure for NBRI16716A (1), NBRI16716B (2) and NBRI16716C (3)
The culture broth (12 l) was filtered and the filtrate was passed through a DIAION HP20 column (800 ml; Mitsubishi Chemical Corporation, Tokyo, Japan) pre-equilibrated with H2O. After washing with H2O (2 l), the active materials were eluted with 25% MeOH (2 l). The eluate was concentrated in vacuo to remove MeOH and diluted with H2O up to 2 l and then extracted with BuOH. The organic layer was concentrated in vacuo to afford 4.1 g of dried materials. The materials were applied on a silica gel column (200 g, Wakogel C-200, 75–150 μm; Wako Pure Chemical Industries, Tokyo, Japan) prepared with CHCl3, and eluted with CHCl3 and CHCl3-MeOH. The fractions eluted with CHCl3-MeOH (2:1) were concentrated in vacuo to give 0.93 g of crude materials. The crude materials were purified by a reversed-phase HPLC column (Inertsil ODS-3, 20 × 250 mm, 6.0 ml min–1; GL Sciences Inc., Tokyo, Japan) with 20% MeOH containing 0.1% TFA to afford crude 543.8 mg of 1 and crude 42.2 mg of 2. The crude samples were applied on gel filtration chromatography of Sephadex LH-20 (50% MeOH; GE Healthcare, Tokyo, Japan) to afford pure 38.5 mg of 1, 31.1 mg of 2 and a trace amount of 3. To obtain more 3, we repeated the fermentation process three times and finally obtained 1.4 mg of 3.
Physico-chemical property
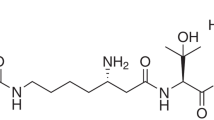
The physico-chemical properties of 1, 2 and 3 are summarized in Table 1. Compounds 1, 2 and 3 were obtained as white powder, which was soluble in MeOH, DMSO and water, but insoluble in CHCl3 and EtOAc. The characteristic color reaction to FeCl3 indicated that they were a family of siderophore.9 The molecular formulae of 1, 2 and 3 were determined to be C18H30N4O8, C18H30N4O7 and C18H30N4O7 by HR-ESI-MS, respectively. The general features of their UV and NMR spectra resembled each other, indicating structural similarities of these compounds.
Structure determination
The NMR spectra of 2 indicated that 2 was identical to the known cleavage fragment of isotriornicin isolated from Epicoccus purpurascens10 as shown in Figure 1. The molecular formula of 1 was determined to be C18H30N4O8, which was one more oxygen atom than that of 2. By comparing NMR data of 1 with those of 2, it was apparent that 1 closely resembled 2 except for the 13C chemical shifts of C-2, C-5, C-6 and C-7. In the 1H NMR spectrum, compound 1 showed only one amide signal at δH 8.30, indicating the replacement of NH to N-OH. The connectivity between proton and carbon atoms was established by the 13C-1H HMQC spectra as shown in Table 2. The 1H-1H COSY and HMBC correlations of 1 are summarized in Figure 2. In the 1H-1H COSY spectrum, sequential proton networks were observed from NH-4 to H-15 through H-3, H-13 and H-14, and from H-6 to H-9. In the HMBC spectrum, cross peaks were observed from NH-4 to C-2 and C-6, from H-7 to C-5 and C-6, from H-13 to C-3 and C-2 and from H-3 to C-2, respectively. Thus, the planar structure of 1 was determined as shown in Figure 1.
Compound 3 was less one oxygen atom than that of 1. The 1H and 13C NMR spectra were very similar to those of 1 except that 3 showed an additional amide signal at δH 7.80 in the 1H NMR spectrum. By comparing various NMR spectra of 3 with those of 1, the difference between 1 and 3 was ascribed to the replacement of N-OH in 1 with NH in 3 at N-16 position.
Compounds 1, 2 and 3 showed optical rotations of −19.6°, −21.3° and −19.2 °, respectively. Three compounds possess asymmetric carbons at C-3 and C-6. The optical rotation of 2 is very close to that of reported value [α]D=−22.95 ° (c 0.78, MeOH)10 indicating the same absolute configuration at C-3 (S) and C-6 (S). Accordingly, the absolute configuration of C-3 and C-6 in 1 and 3 was considered to be identical with 2 based on the optical rotations and biosynthetic aspect. As the 13C-chemical shifts of C-22 in three compounds were almost identical to that of reported values of 2,10 the double bonds of C-18 in 1, 2 and 3 were assigned to E-configuration.
Biological activities
The effects of 1, 2 and 3 on coculture of human prostate cancer DU-145 cells with PrSCs were determined using rhodanile blue staining method.5 The coculture system shows the tumor–stromal cell interactions of prostate cancer, in which the growth of DU-145 cells is increased by the coculture with PrSCs.3, 5 As shown in Figure 3, although both 1 and 2 moderately inhibited the growth of DU-145 cells cultured alone with IC50 values at almost 100 μg ml–1, both compounds inhibited the growth of DU-145 cells in coculture with PrSCs more strongly than that of DU-145 cells alone with IC50 values at approximately 10 μg ml–1. In contrast, compound 3 equally inhibited the growth of DU-145 cells in both culture conditions with IC50 values at almost 100 μg ml–1. All three compounds did not show apparent cytotoxicity against PrSCs under microscopic observation (data not shown).
Effects of 1, 2 and 3 on coculture of DU-145 cells and PrSCs. The growth of DU-145 cells cocultured with PrSCs (closed symbols) or that of DU-145 cells alone (open symbols) in the presence of the indicated concentrations of 1 (circles), 2 (squares) or 3 (triangles) was determined using rhodanile blue method. Values are means of duplicate determinations. Each s.e. is <10%.
Acute toxicity of 1 and 2 in mice was examined using ICR female mice. When both 1 and 2 were administered intravenously, intraperitonealy or orally, no fatality was observed up to 100 mg kg–1. We then examined the antitumor effects of both compounds against xenograft models. We inoculated DU-145 cells with PrSCs into nude mice subcutaneously and then injected 1 and 2 intraperitonealy. As a result, both compounds apparently inhibited the growth of tumors of DU-145 cells and PrSCs in vivo without any effect on body weight (Figure 4).
Effects of 1 and 2 on tumor growth of DU-145 cells in vivo. DU-145 cells were inoculated subcutaneously with PrSCs in female nude mice. Compound 1 (•) or 2 (▪) was administered intraperitonealy at 50 mg kg–1 on days 17–19, 21–26, 28–33 and 35–40. The values are means±s.d. of five mice. *P<0.05 versus the values with saline (○).
Compounds 1 and 2 did not show antimicrobial and antifungal activities at 100 μg ml–1.
Discussion
Because compound 2 was originally reported as an artificial fragment of isotriornicin cleaved by methanol-ammonia,10 it is a very curious fact that we obtained it as a natural product. Although isotriornicin was isolated from E. purpurascens,10 our compounds were isolated from the different fungus, P. melioloides. The biological effects of the fragment and isotriornicin were not reported, but it is interesting that triornicin, an isomer of isotriornicin, has been reported to possess slight antitumor activity against Ehrlich ascites tumor in vivo.9, 11 It is interesting that 3 unexpectedly could not exert differential growth inhibitory activity similar to 1 and 2 (Figure 3), suggesting that N-hydroxyamide moiety at N-16 might be critical to the activity. Although the mechanisms of actions of 1 and 2 as well as that of triornicin are still unknown, they might have a similar target. We are now studying the precise mechanism of antitumor effects of 1 and 2.
We have recently reported that leucinostatins and atpenins showed antitumor effect against tumors of DU-145 cells and PrSCs in vivo.8 These compounds were also re-discovered as modulators of tumor–stromal cell interactions by our screening program.5, 6 In this paper, we have reported new natural compounds from fungal metabolite. Although the study of the precise mechanism is going on, 1 and 2 are considered to modulate the tumor–stromal cell interactions similar to other compounds reported previously.6, 7, 8 As we obtained a new compound with potency of antitumor effect in vivo, we thought that our screening concept and program would be beneficial for the innovation of new antitumor drugs.
Methods
Reagents
Rhodanile blue was purchased from Aldrich (Milwaukee, WI, USA). Insulin and hydrocortisone were obtained from Sigma (St Louis, MO, USA). Transferrin was obtained from Wako Pure Chemical Industries. The recombinant human basic fibroblast growth factor was purchased from Pepro Tech (London, UK).
Cells
The human prostate cancer DU-145 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (ICN Biomedicals, Aurora, OH, USA), 100 units ml–1 penicillin G and 100 μg ml–1 streptomycin at 37 °C with 5% CO2. The human normal PrSCs were obtained from Bio Whittaker (Walkersville, MD, USA) and maintained in DMEM supplemented with 10% fetal bovine serum, 100 units ml–1 penicillin G, 100 μg ml–1 streptomycin, ITH (5 μg ml–1 insulin, 5 μg ml–1 transferrin and 1.4 μM hydrocortisone) and 5 ng ml–1 basic fibroblast growth factor at 37 °C with 5% CO2.
Coculture experiment
A microplate assay method for the selective measurement of epithelial tumor cells in coculture with stromal cells using rhodanile blue dye was performed as described before.5 The PrSCs were first inoculated into 96-well plates at 5000 cells per well in 100 μl of DMEM supplemented with ITH and 0.1% fetal bovine serum in the presence of the various concentrations of test compounds. After 2 days, 10 μl of DU-145 cell suspension (5000 cells) in serum-free DMEM was inoculated onto a monolayer of PrSCs, and the cells were further cultured for 3 days. For monoculture of DU-145 cells, the assay medium alone was first incubated in the presence of test compounds for 2 days at 37 °C. Then, DU-145 cells were inoculated as described above, and cultured for further 3 days. The growth inhibitory activity was expressed as percentage of cell number compared with control cell number as 100%.
Antitumor effect in vivo
Female nude mice, 6 weeks old, were purchased from Charles River Breeding Laboratories (Yokohama, Japan) and maintained in a specific pathogen-free barrier facility according to our institutional guidelines. DU-145 cells (8 × 106) were trypsinized and resuspended with or without PrSCs (8 × 106) in 0.3 ml of 10% fetal bovine serum/DMEM and then combined with 0.5 ml of growth factor-reduced Matrigel (BD Biosciences, San Jose, CA, USA). A total of 100 μl of the cell suspension (1 × 106 cells) was injected subcutaneously in the left lateral flank of mice. Five mice were used for each experimental set. Tumor volume was estimated using the following formula: tumor volume (mm3)=(length × width2)/2. After the indicated times, tumors were surgically dissected.
Analytical measurement
Optical rotations were measured on a JASCO P-1030 polarimeter (JASCO, Tokyo, Japan). UV spectra were recorded on a Hitachi 228 A spectrometer (Hitachi, Tokyo, Japan). 1H and 13C NMR spectra were measured on a JEOL JNM A400 spectrometer (JEOL, Tokyo, Japan) using TMS as an internal standard. HRESI-MS spectra were measured with a JEOL JMS-T100LC spectrometer (JEOL).
Fermentation
Fungal strain, P. melioloides Mer-f16716, was isolated from a soil sample collected from Yakushima Island, Kagoshima prefecture, Japan. The sequence analysis of partial 28S rRNA (556 nucleotides) of this strain showed high identity with P. melioloides (99.3%). Thus, the strain Mer-f16716 was tentatively identified as a member of P. melioloides. The strain grown on an agar slant was inoculated into a 250-ml flask containing 25 ml of a seed medium consisting of 2% potato starch, 1% glucose, 2% Soypro (J-Oil Mills, Tokyo, Japan), 0.1% KH2PO4, 0.05% MgSO4 7H2O and three glass beads, and cultured at 25 °C for 3 days on a rotary shaker at 220 r.p.m. Seed culture (1 ml) was inoculated into a 500-ml flask containing 60 ml of a culture medium consisting of 4% dextrin hydrate, 1% Bacto Peptone (BD Biosciences), 2% Pharmamedia (Traders Protein, Memphis, TN, USA), 0.5% Extract Ehlrich (Wako Pure Chemical Industries), 0.29% NaCl, 0.06% MgSO4 7H2O, 0.05% MgCl2 6H2O, 1% mineral solution (solution of 0.725% KCl, 0.097% KBr, 0.026% H3BO3, 0.00395% KF, 0.00008% KI, 0.001475% CaCl2 2H2O and 0.0215% SrCl2 6H2O) (adjusted at pH 7.2 before sterilization) and cultured at 25 °C for 4 days on a rotary shaker at 220 r.p.m.
References
Grossfeld, G. D., Hayward, S. W., Tlsty, T. D. & Cunha, G. R. The role of stroma in prostatic carcinogenesis. Endocr. Relat. Cancer 5, 253–270 (1998).
Tuxhorn, J. A., Ayala, G. E. & Rowley, D. R. Reactive stroma in prostate cancer progression. J. Urol. 166, 2472–2483 (2001).
Kawada, M., Inoue, H., Masuda, T. & Ikeda, D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 66, 4419–4425 (2006).
Kawada, M., Inoue, H., Arakawa, M. & Ikeda, D. Transforming growth factor-β1 modulates tumor-stromal cell interactions of prostate cancer through insulin-like growth factor-I. Anticancer Res. 28, 721–730 (2008).
Kawada, M. et al. A microplate assay for selective measurement of growth of epithelial tumor cells in direct coculture with stromal cells. Anticancer Res. 24, 1561–1568 (2004).
Kawada, M., Momose, I., Someno, T., Tsujiuchi, G. & Ikeda, D. New atpenins, NBRI23477 A and B, inhibit the growth of human prostate cancer cells. J. Antibiot. 62, 243–246 (2009).
Kawada, M., Inoue, H., Usami, I. & Ikeda, D. Phthoxazolin A inhibits prostate cancer growth by modulating tumor-stromal cell interactions. Cancer Sci. 100, 150–157 (2009).
Kawada, M. et al. Leucinostatin A inhibits prostate cancer growth through reduction of insulin-like growth factor-I expression in prostate stromal cells. Int. J. Cancer 126, 810–818 (2010).
Frederick, C. B., Szaniszlo, P. J., Vickrey, P. E., Bentley, M. D. & Shive, W. Production and isolation of siderophores from the soil fungus Epicoccum purpurascens. Biochemistry 20, 2432–2436 (1981).
Frederick, C. B., Bentley, M. D. & Shive, W. The structure of the fungal siderophore, isotriornicin. Biochem. Biophys. Res. Commun. 105, 133–138 (1982).
Frederick, C. B., Bentley, M. D. & Shive, W. Structure of triornicin, a new siderophore. Biochemistry 20, 2436–2438 (1981).
Acknowledgements
We are grateful to Dr K Isshiki and Dr N Sakata (Mercian Corporation Bioresource Laboratories) for their valuable discussions. We also thank Dr R Sawa and Ms Y Kubota (Microbial Chemistry Research Center) for analysis of HRESI-MS and NMR measurements, and Ms K Adachi and Ms E Satoh for their technical assistance. This work was supported in part by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawada, M., Someno, T., Inoue, H. et al. NBRI16716A, a new antitumor compound against human prostate cancer cells, produced by Perisporiopsis melioloides Mer-f16716. J Antibiot 63, 319–323 (2010). https://doi.org/10.1038/ja.2010.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2010.42
Keywords
This article is cited by
-
Novel approaches for identification of anti-tumor drugs and new bioactive compounds
The Journal of Antibiotics (2018)
-
Notes for genera: Ascomycota
Fungal Diversity (2017)
-
Small molecules modulating tumor–stromal cell interactions: new candidates for anti-tumor drugs
The Journal of Antibiotics (2016)
-
Intervenolin, a new antitumor compound with anti-Helicobacter pylori activity, from Nocardia sp. ML96-86F2
The Journal of Antibiotics (2013)