Abstract
The WalK (a histidine kinase)/WalR (a response regulator, aka YycG/YycF) two-component system is indispensable in the signal transduction pathway for the cell-wall metabolism of Bacillus subtilis and Staphylococcus aureus. The inhibitors directed against WalK would be expected to have a bactericidal effect. After we screened 1368 culture broths of Streptomyces sp. by a differential growth assay, walkmycin A, B and C, which were produced by strain MK632-100F11, were purified using silica-gel column chromatography and HPLC. In this paper, the chemical structure of the major product (walkmycin B) was determined to be di-anthracenone (C44H44Cl2O14), which was very similar to BE40665A. MICs of walkmycin B against B. subtilis and S. aureus were 0.39 and 0.20 μg ml−1, and IC50 measurements against WalK were 1.6 and 5.7 μM, respectively. To clarify the affinity between WalK and walkmycin B, surface plasmon resonance was measured to obtain the equilibrium dissociation constant, KD1, of 7.63 μM at the higher affinity site of B. subtilis WalK. These results suggest that walkmycin B inhibits WalK autophosphorylation by binding to the WalK cytoplasmic domain.
Similar content being viewed by others
Introduction
In the past decade, development of bacterial genomics, bioinformatics and gene manipulation have led to the discovery of many novel protein targets for antibacterial agents.1 For instance, the two-component signal transduction systems of bacteria, which consist of two proteins, histidine kinase (HK) and response regulators, have received increasing attention on account of their potential as novel antibacterial drug targets.2, 3, 4
Earlier work has reported on five chemotypes (cyclohexene, closantel, benzimidazole, trityl and bisphenol) that possess inhibitory activity against KinA (an HK with an IC50 value ranging from 2 to 20 μM) and that result in antibacterial activity.5 These compounds also had an appreciable effect on the cell membrane integrity or caused hemolysis of equine erythrocytes. Although they were inhibitors against non-essential HKs, no absolute proof has been presented showing that growth inhibition was the direct consequence of HK inhibition.
The WalK/WalR (aka YycG/YycF) two-component signal transduction system has appeared as a promising candidate, as it is essential for bacterial survival and is specific to low G+C% Gram-positive bacteria, including Bacillus subtilis, Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis and S. pyogenes.6, 7, 8, 9, 10 Recent studies have unveiled a conserved function for this system in different bacteria, defining this signal transduction pathway as a master regulatory system for cell-wall metabolism, owing to which YycG/YycF has been accordingly renamed as WalK/WalR.11, 12 Thus, inhibitors directed against WalK/WalR may have a bactericidal effect and may be active against an array of clinically important Gram-positive pathogens.
WalK is a membrane-linked kinase, with a conserved C-terminal cytoplasmic HK region, including ATP binding and phosphoacceptor domains. Sequence similarities indicate that WalK phosphorylation occurs on conserved residue His387(all amino acid sequence coordinates are those of the prototypical B. subtilis proteins), although the aminoterminal domain of the WalK HK shows considerable variation among species. The conserved C-terminal cytoplasmic HK region can be used for screening WalK inhibitors.
First, inhibitors targeting WalK (C-terminal cytoplasmic HK region) of B. subtilis were analyzed to obtain imidazole derivatives (NH125).13 Imidazole derivatives had antibacterial activity in drug-resistant S. aureus, E. faecalis and S. pneumoniae, as well as in B. subtilis, with MICs of 0.39–6.25 μg ml−1. However, the inhibitors have caused structural alteration of HK leading non-specifically to aggregation.
The specific hypersensitivities shown by temperature-sensitive mutants indicate that use of these mutants in whole-cell screening provides a rapid method to develop target-specific screens for the identification of novel compounds.14, 15 Previously, we developed a differential growth assay targeting WalK/WalR two-component signal transduction systems, in which a temperature-sensitive walR mutant (CNM2000) of B. subtilis was supersensitive to inhibitors of HK, in comparison with the wild-type 168 strain.16, 17 As a result, an antibacterial agent, aranorosinol B, was isolated as an HK inhibitor by this differential growth assay.16, 17 To isolate more active inhibitors targeting WalK, a wide range of soil microbacteria culture extracts were further screened by this method, resulting in the acquisition of a potent WalK inhibitor.
Materials and Methods
Strains and plasmids
The strains and plasmids used in this study are shown in Table 1.
Construction of pETYycG-tru
The pETYycG-tru was constructed using the following method. With pBY33 as the template and (5′-GGTATTTTTCTGGCGGGATCCCGTACCCAC-3′) and (5′-TATCGTTTTTAGCGGCCGCCGCTTCATCCC-3′) as the primers, the sequence that encodes the cytoplasmic domain (207–611 amino acids) of WalK of B. subtilis (WalK (Bs)) was amplified by polymerase chain reaction using Ex Taq DNA polymerase (Takara Bio, Shiga, Japan). This polymerase chain 7reaction fragment was inserted between the restriction enzyme sites BamHI and NotI of expression vector pET21-a (+) (Novagen, Madison, WI, USA) using Ligation kit Ver.2.1 (Takara Bio).
Purification of WalK
The cytoplasmic domain (C-terminus His-tag) of WalK of B. subtilis (WalK (Bs)) and S. aureus (WalK (Sa)) was expressed and purified using Escherichia coli BL21 (DE3) containing pETYycG-tru and pYycGSa,16 respectively. They were grown in 2 × YT culture (1.6% Bacto tryptone (Difco, Franklin Lakes, NJ, USA), 1% Yeast extract (Difco), 0.5% NaCl) at 30 °C. When OD600 was ∼0.6, IPTG (final concentration 0.5 mM) was added and incubated for 3 h. Then, the bacterial cells were disrupted by sonication and centrifuged. The supernatant was subjected to affinity chromatography using Ni–NTA agarose (Qiagen, Los Angeles, CA, USA) for purification. After solvent displacement by dialysis in a storage buffer (50 mM Tris-HCl (pH 7.6), 200 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 1 mM dithiothreitol and 50% glycerol), they were stored at −20 °C. The purified cytoplasmic domains of WalK (Bs) and WalK (Sa) were used as WalK in this study.
Differential growth assay
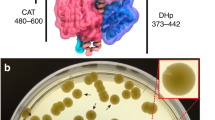
Wild-type parent 168 and CNM2000 strains were each cultured overnight in 3% Trypticase soy broth (Becton Dickinson, Franklin Lakes, NJ, USA). Then, 30 μl of the overnight culture solution was added to 3 ml of top agar (0.75% Trypticase soy broth, 1.5% agar) dissolved at 55 °C, and the solution was poured into a 10-ml bottom agar plate (0.75% Trypticase soy broth, 0.5% agar). After sufficient cooling, 1 μl of the screen sample was spotted. After overnight culture at 37 °C, halos were observed in the 168 plate and the CNM 2000 plate (Figures 1b and 3).
(a) HPLC analysis. Cell culture of MK632-100F11 was centrifuged and the pellet was extracted with methanol, concentrated and chromatographed on column of Sephadex LH-20. (b) Sensitivity of B. subtilis 168 and CNM2000 to walkmycins; 1 μl of walkmycin A, B and C (1000, 250 and 62.5 μg ml−1) was spotted on agar plates containing B. subtilis 168 (left) or CNM2000 (right) with differential growth assay.
Spectroscopic analysis
The optical rotation of the isolated walkmycins was measured with a JASCO (Tokyo, Japan) P-1030 polarimeter. UV spectra were recorded with a Hitachi U-2800 spectrophotometer (Hitachi, Tokyo, Japan). IR spectrum was recorded with a Horiba FT-210 Fourier transform infrared spectrometer (Horiba, Kyoto, Japan). The 1H and 13C NMR spectra were measured with a JEOL JNM-ECA600 spectrometer at 25 °C using TMS as an internal reference. The mass spectrum was recorded with a Thermo Fisher Scientific (Waltham, MA, USA) LTQ Orbi trap XL mass spectrometer.
Inhibition of WalK autophosphorylation
In all, 2 μl of WalK solution (2.5 pmol μl−1 of WalK (Bs) and WalK (Sa)) and 5 μl of 2 × kinase buffer (100 mM Tris-HCl (pH 8.5), 200 mM KCl, 200 mM NH4Cl, 10 mM MgCl2) were mixed. Then, 1 μl of drug solution was added. After 5 min, 2 μl of 12.5 μM ATP solution ([γ-32P] ATP 18.5 kBq μl−1) was added for autophosphorylation. The reaction was stopped 10 min later using a 2 × SDS sample buffer (20 mM Tris-HCl, pH 6.8, 20% glycerol, 4% SDS, 10% β-mercaptoethanol, 0.1% bromophenol blue) and SDS–polyacrylamide gel electrophoresis was performed. After drying, the gel was exposed to an imaging plate. Signals were detected by FLA-7000 (Fuji Photo Film, Tokyo, Japan) using the Multi Gauge Ver. 3.0 (Fuji Photo Film) analytical software, and Prism 5 (GraphPad Software, La Jolla, CA, USA) was used to calculate the IC50 (50%).
Antimicrobial activity
MICs were determined by a standard agar dilution method recommended by the Japan Society of Chemotherapy.18 The bacteria were incubated in a Mueller–Hinton agar (Difco) at 37 °C for 18 h, while yeast was incubated for 42 h.
Surface plasmon resonance measurements
The binding affinity of walkmycin B toward WalK (Bs) was assayed with SensiQ Pioneer (ICx Nomadics, Oklahoma City, OK, USA) at 25 °C. The running buffer for the system was 10 mM HEPES (pH 7.4), 150 mM NaCl, 2 mM EDTA, 0.005% (v/v) Tween-20 and 2% (v/v) methanol. WalK (Bs) was immobilized on the surface of a COOH1 sensor chip, according to the manufacturer's manual. Briefly, the surface of channel 1 on the chip was activated by injecting a mixture of 0.2 M EDC and 0.05 M NHS for 2 min at a flow rate of 50 μl min−1. Next, WalK (Bs) solution (500 μg ml−1) in a 10 mM acetate buffer (pH 4.3) was injected for 10 min over channel 1. To increase the immobilized yield, WalK (Bs) solution (diluted ∼1:36 with the EDC/NHS mixture) was further injected for 16 min. Finally, to cap the remaining NHS esters, 1 M ethanolamine (pH 8.0) was injected for 4 min. The other channels were left unmodified to serve as references. To obtain a dose-dependent response curve set for walkmycin B, a twofold dilution series was prepared in the running buffer, ranging in concentration from 0.451 to 57.7 μM of walkmycin B. Each sample was injected for 3 min at a flow rate of 50 μl min−1 in duplicate. Dissociation of the compound was monitored for 20 min to ensure full removal from the surface. Running buffer blanks were included to subtract any systematic artifact. To determine the dissociation and kinetic constants of the walkmycin B binding from the response curve set, a protein–ligand binding model at 1:2 molar ratio (see Results and Discussion) was applied as described in Supplementary Information.
Results and Discussion
Screening and identification of strain MK632-100F11
Methanol extracts from 1368 microbes were used for differential growth assay.17 One sample from the strain MK632-100F11 showed stronger activity against CNM2000 than strain 168. Strain MK632-100F11 was isolated from a soil sample collected at Hokuto City, Yamanashi Prefecture, Japan. The morphological, cultural and physiological properties of MK632-100F11 were examined according to the methods described by Shirling and Gottlieb19 and Waksman.20 Strain MK632-100F11 produced well-branched vegetative mycelia and formed long and straight aerial hyphae. The mature spore chain consisted of 10–50 spores. The spore was oval with a smooth surface and 0.5–0.6 × 1.2–1.7 μm in size. No synnemata, sclerotia or sporangia were observed. The aerial mycelia of strain MK632-100F11 were brownish white to light brownish gray on various agar media. The substrate mycelia were pale yellow to yellow. The soluble pigments were produced as yellowish pigment. Permissive temperature ranges for growth of the strain were 16–37 °C. The optimal temperature for growth of strain MK632-100F11 was 27–30 °C. Whole-cell hydrolysates of strain MK632-100F11 contained LL-diaminopimelic acid.21 The 16S ribosomal RNA gene (16S rDNA) was amplified by polymerase chain reaction using genomic DNA of strain MK632-100F11 and sequenced. A homology search of the most related sequences was performed using the BLAST algorithm on the DDBJ/Genebank/EMBL. The partial 16S rDNA sequence (1459 bp) of strain MK632-100F11 showed high identity value with genus Streptomyces, such as S. pseudovenezuelae NBRC 12904T 1445/1456 bp, 99.2%; S. novaecaesareae NBRC13368T 1444/1457 bp, 99.1%; S. galilaeus JCM4757T 1447/1465 bp, 98.8%; and S. flavovariabilis NRRLB-16367T 1449/1468 bp, 98.7%. From the phenotypic characteristics, strain MK632-100F11 was closely related to the species of S. pseudovenezuelae.22 These taxonomical properties suggested that strain MK632-100F11 belonged to the genus Streptomyces. Therefore, strain MK632-100F11 was designated Streptomyces sp. MK632-100F11.
Purification of walkmycin B
A slant culture of MK632-100F11 was inoculated into a 500 ml baffled Erlenmeyer flask containing 110 ml of a seed medium consisting of galactose 2.0%, dextrin 2.0%, Bacto-soytone (Difco) 1.0%, corn steep liquor (Iwaki, Tokyo, Japan) 0.5%, glycerol 1.0%, (NH4)2SO4 0.2% and CaCO3 0.2% in deionized water (pH 7.4 before sterilization). The culture was incubated in a rotary shaker (180 r.p.m.) at 30 °C for 2 days. The seed culture of the strain was transferred into a 500 ml baffled Erlenmeyer flask containing 110 ml of a producing medium consisting of glycerol 2.0%, dextrin 2.0%, Bacto-soytone (Difco) 1.0%, yeast extract 0.3%, (NH4)2SO4 0.2% and CaCO3 0.2% in deionized water (pH 7.4). The fermentation was carried out at 27 °C for 5 days on a rotary shaker (180 r.p.m.). The fermentation broth (5 l) was separated to the mycelial cake and supernatant by centrifugation. The mycelial cake was extracted with 2.7 l of MeOH. The MeOH extract was filtered and concentrated in vacuo. The active principle was added to 2 l water and extracted with 2.5 l of EtOAc. The EtOAc solution was concentrated under reduced pressure to yield 2 g of dark brown oil containing walkmycin B and trace amounts of related compounds (walkmycin A and C). The concentrate was chromatographed on a column of Sephadex LH-20 (ϕ 36 × 480 mm, GE Healthcare, Uppsala, Sweden) developed with MeOH. Three active fractions were detected by the differential growth assay (Figures 1a and b); namely, walkmycin A, B and C. Growth of CNM2000 was more sensitive to walkmycin A, B and C than that of the wild-type strain 168 as shown in Figure 1b. In this paper, we describe only about the walkmycin B fraction; the details of walkmycin A and C should be published elsewhere. Walkmycin B was concentrated in vacuo to give 99.7 mg of brownish yellow oil. The crude oil was further purified by crystallization in a mixture solvent of CHCl3–MeOH (3:10) at 5 °C to yield 44.8 mg of the oil as fine yellow microcrystalline. The physico-chemical properties of walkmycin B are as follows: [α]D23−195.8° (c 1.0, acetone); HR-MS (ESI, positive): m/z 867.2180 (M+H)+ (calcd. for C44H45Cl2O14, 867.2181); UV λmax MeOH (ɛ): 235 (56 200), 279 (57 600), 331 (9700), 425 (22 000); IR νmax (KBr, cm−1): 3700–3200, 2974, 2943, 1691,1614, 1587 (sh), 1400, 1375, 1313, 1220, 1196, 1167; 1H NMR (600 MHz, CDCl3:CD3OD=4:1) δ: 0.54 (6H, d, J=7 Hz), 1.17 (6H, d, J=7 Hz), 1.43 (6H, d, J=7 Hz), 2.17 (2H, m), 3.42 (2H, q, J=7), 3.57 (6H, s), 5.43 (2H, s), 6.1 (2H, s), 7.77 (2H, d, J=9 Hz), 7.84 (2H, d, J=9 Hz); 13C NMR (150 MHz, CDCl3:CD3OD=4:1) δ: 8.00 × 2 (q), 17.50 × 2 (q), 20.30 × 2 (q), 35.90 × 2 (d), 45.60 × 2 (d), 48.70 × 2 (d), 56.70 × 2 (q), 77.60 × 2 (s), 94.50 × 2 (d), 111.9 × 2 (s), 114.2 × 2 (s), 115.7 × 2 (d), 121.0 × 2 (s), 121.1 × 2 (s), 131.6 × 2 (s), 135.8 × 2 (s), 135.9 × 2 (d), 155.6 × 2 (s), 162.6 × 2 (s), 173.0 × 2 (s), 173.4 × 2 (s), 206.7 × 2 (s).
The molecular formula of walkmycin B was established as C44H44Cl2O14 on the basis of HRESI-MS. The chemical shifts of 1H and 13C NMR, and physico-chemical properties of walkmycin B were very similar to those of BE40665 A.23 As walkmycin B is not stable in DMSO and acetone, we could not compare the chemical shifts of 1H, 13C NMR and the specific rotation of walkmycin B with those of BE40665 A. However, a series of two-dimensional NMR and nuclear overhauser enhancement spectroscopy analyses indicated the structure of BE40665 A. As a result, walkmycin B was closely related to BE40665 A (Figure 2). Detailed stereochemical studies of walkmycin B are now in progress.
Biological activity
Walkmycin B, NH125 (a non-selective HK inhibitor) and a panel of antibiotics were subjected to the differential growth assay, to seek how they affect the growth of CNM2000 and wild-type 168. The results showed that Walkmycin B is the only compound to show enhanced activity toward CNM2000 relative to wild type (Figure 3). When B. subtilis 168 was incubated in the presence of walkmycin B (0.25–1.0 μg ml−1), the cells also lost their cytoplasmic content and only the cell wall remained and resulted in ghost (see the arrowhead in Figure 4) as reported earlier.6, 24 Furthermore, the results of MIC measurement showed that for Gram-positive bacteria having WalK (B. subtilis, S. aureus and E. faecalis) (Table 2), antibacterial activity was shown in the MIC range of 0.20–6.25 μg ml−1. However, for Gram-negative bacteria and other microbes without the WalK/WalR signal transduction system, no clear antibacterial activity was shown at MIC ⩾100 μg ml−1. These results strongly suggest that walkmycin B inhibits the WalK/WalR signal transduction system and shows antibacterial activity.
Sensitivity of B. subtilis 168 and CNM2000 to antibacterial agents; 1 μl of antibacterial agents (50 μg ml−1) was spotted on agar plates containing B. subtilis 168 (left) or CNM2000 (right) as described in ‘Materials and methods’ (differential growth assay): walkmycin B (1), NH125 (2), ampicillin (3), vancomycin (4), amikacin (5), erythromycin (6), ofloxacin (7), rifampicin (8). NH125 is a non-specific HK inhibitor.13
Effect of walkmycin B on cell morphology. After B. subtilis 168 was incubated in the absence (photo 1) or presence of walkmycin B (photo 2, 0.25 μg ml−1; photo 3, 0.5 μg ml−1; photo 4, 1.0 μg ml−1) in LB for 4 h at 37 °C, it was observed under a microscope ( × 800). The arrow indicates the ghost cell.6, 24
HK inhibitory activity
To determine the stage of the WalK/WalR signal transduction system at which walkmycin B acts, the inhibitory activity of walkmycin B against WalK autophosphorylation was assayed. As shown in Figure 5, the bands for the autophosphorylated WalK (Bs) and WalK (Sa) clearly disappeared depending on the increase in walkmycin B concentration. The half maximal IC50 against WalK (Bs) and WalK (Sa) were calculated to be 1.6 and 5.7 μM, respectively, by densitometrically quantifying the autophosphorylation bands.
Specific binding of walkmycin B
To investigate the WalK (Bs)–walkmycin B interaction, a surface plasmon resonance-based assay was performed with SensiQ Pioneer (ICx Nomadics) equipped with the COOH1 chip. The surface of the COOH1 chip is composed of carboxylated polyethylene glycol so that it resists non-specific binding of most biological molecules. In the surface plasmon resonance experiments, ∼450 response units of WalK (Bs) were immobilized to channel 1 of the sensor surface (data not shown). Figure 6 shows the overlaid responses of the immobilized WalK (Bs) for walkmycin B. The duplicate responses for each concentration show that the system exhibited good reproducibility with negligible degradation of the protein. Regeneration of the chip surface was not necessary as the compound fully dissociated from the surface within 20 min. The response unit value increased with the increase in walkmycin B concentration, which indicated that walkmycin B was able to bind to WalK (Bs) in a dose-dependent manner. Walkmycin B at 57.7, 28.9 and 14.4 μM yielded significant responses, allowing detailed kinetic analysis. First, the simple 1:1 kinetic model was fitted to the response data, but the curve fitting was unsuccessful for the responses. In particular, the theoretical line significantly deviated from the observed data points at the dissociation phase. Then, a 1:2 molar ratio protein–ligand binding model was applied to the fitting (see Supplementary Information), by virtue of which walkmycin B associates with two binding sites and dissociates from them in a fully independent manner. As shown in Figure 6, the theoretical line (red) in this fitting model showed good agreement with the observed response curves for walkmycin B at 57.7, 28.9 and 14.4 μM. In the best fitting, the association rate constant (ka1) was 101 M−1s−1 and the dissociation rate constant (kd1) was 7.71 × 10−4 s−1, and the response max of the compound was 6.9 response units and the affinity constant (KD1) of the interaction was 7.63 μM for the high-affinity site. For the lower-affinity site, ka2, kd2, response max and KD2 were 461 M−1 s−1, 1.79 × 10−2 s−1, 8.2 and 38.9 μM, respectively. These results show that walkmycin B specifically binds to WalK (Bs) with relatively high affinity and dissociates from WalK (Bs) reversibly. The estimated contributions of the two independent binding sites to the surface plasmon resonance response (6.9 and 8.2 response units for high- and low-affinity sites, respectively) are comparable, strongly supporting the notion that two binding sites of walkmycin B are located on WalK (Bs). Although the experimental conditions are not identical, the KD1 value of the higher-affinity site for walkmycin B is highly similar to its IC50 value (1.6 μM) determined by the autophosphorylation inhibition assay, suggesting that the walkmycin B binding to the higher affinity site of WalK (Bs) is relevant for the inhibition of WalK autophosphorylation.
Surface plasmon resonance measurement and specific binding of walkmycin B. The duplicated response curves were obtained from injections of series of concentrations of walkmycin B over WalK (Bs) immobilized on the COOH1 chip with SensiQ Pioneer (ICx Nomadics). The walkmycin B concentrations (μM) are shown with the responses. The details of measurement are described in the text. The theoretical curves (red) calculated from the best fit parameters are shown with the observed experimental data (black). A full-color version of this figure is available at The Journal of Antibiotics online.
References
Moir, D. T., Shaw, K. J., Hare, R. S. & Vovis, G. F. Genomics and antimicrobial drug discovery. Antimicrob. Agents Chemother. 43, 439–446 (1999).
Barrett, J. F. & Hoch, J. A. Two-component signal transduction as a target for microbial anti-infective therapy. Antimicrob. Agents Chemother. 42, 1529–1536 (1998).
Macielag, M. J. & Goldschmidt, R. Inhibitors of bacterial two-component signaling systems. Expert Opin. Investig. Drugs 9, 2351–2369 (2000).
Matsushita, M. & Janda, K. D. Histidine kinases as targets for new antimicrobial agents. Bioorg. Med. Chem. 10, 855–867 (2002).
Hilliard, J. J., Goldschmidt, R. M., Licata, L., Baum, E. Z. & Bush, K. Multiple mechanisms of action for inhibitors of histidine protein kinases from bacterial two-component systems. Antimicrob. Agents Chemother. 43, 1693–1699 (1999).
Fabret, C. & Hoch, J. A. A two-component signal transduction system essential for growth of Bacillus subtilis: implications for anti-infective therapy. J. Bacteriol. 180, 6375–6383 (1998).
Lange, R. et al. Domain organization and molecular characterization of 13 two-component systems identified by genome sequencing of Streptococcus pneumoniae. Gene 237, 223–234 (1999).
Martin, P. K., Li, T., Sun, D. X., Biek, D. P. & Schmid, M. B. Role in cell permeability of an essential two-component system in Staphylococcus aureus. J. Bacteriol. 181, 3666–3673 (1999).
Throup, J. P. et al. A genomic analysis of two-component signal transduction in Streptococcus pneumoniae. Mol. Microbiol. 35, 566–576 (2000).
Hancock, L. & Perego, M. Two-component signal transduction in Enterococcus faecalis. J. Bacteriol. 184, 5819–5825 (2002).
Dubrac, S., Boneca, I. G., Poupel, O. & Msadek, T. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 189, 8257–8269 (2007).
Dubrac, S., Bisicchia, P., Devine, K. M. & Msadek, T. A matter of life and death: cell wall homeostasis and the WalKR (YycGF) essential signal transduction pathway. Mol. Microbiol. 70, 1307–1322 (2008).
Yamamoto, K. et al. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis. Biosci. Biotechnol. Biochem. 65, 2306–2310 (2001).
Numata, K., Yamamoto, H., Hatori, M., Miyaki, T. & Kawaguchi, H. Isolation of an aminoglycoside hypersensitive mutant and its application in screening. J. Antibiot. 39, 994–1000 (1986).
Kamogashira, T. & Takegata, S. A screening method for cell wall inhibitors using a D-cycloserine hypersensitive mutant. J. Antibiot. 41, 803–806 (1988).
Watanabe, T. et al. Isolation and characterization of inhibitors of the essential histidine kinase, YycG in Bacillus subtilis and Staphylococcus aureus. J. Antibiot. 56, 1045–1052 (2003).
Okada, A. et al. Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol. 422, 386–395 (Elsevier Academic Press, San Diego, 2007).
Japanese Society of Chemotherapy. Final report from the Committee on Antimicrobial Susceptibility Testing, Japanese Society of Chemotherapy, on the agar dilution method. Chemotherapy 56, 49–57 (2008).
Shirling, E. B. & Gottlieb, D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 16, 313–340 (1966).
Waksman, S. A. in The Actinomycetes, Vol. 2 (ed. Waksman, S. A., The Williams & Wilkins Co., Baltimore, 1961).
Staneck, J. L. & Roberts, G. D. Simplified approach to identification of aerobic actinomycetes by thin-layer chromatography. Appl. Microbiol. 28, 226–231 (1974).
Skerman, V. B. D., McGowan, V. & Sneath, P. H. A. Approved lists of bacterial names. Int. J. Syst. Bacteriol. 30, 225–420 (1980).
Tsukamoto, T., Nakajima, S ., Suzuki, H., Ojiri, K & Suda, H. Kokai Tokkyo Koho JP 278055 (1995).
Fukuchi, K. et al. The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology 146, 1573–1583 (2000).
Acknowledgements
We thank Dr Masaki Hatano and Miss Yoshiko Homma for fermentation, Mrs Rie Arisaka for purification and Mrs Yumiko Kubota for measuring the NMR spectra. This work was supported by a Grant-in-Aid for Scientific Research (A, 20248012) of the Japan Society for the Promotion of Science (JSPS), and the Research and Development Program for New Bio-Industry Initiatives (2006–2010) of the Bio-Oriented Technology Research Advancement Institution (BRAIN).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on The Journal of Antibiotics website (http://www.nature.com/ja)
Supplementary information
Rights and permissions
About this article
Cite this article
Okada, A., Igarashi, M., Okajima, T. et al. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J Antibiot 63, 89–94 (2010). https://doi.org/10.1038/ja.2009.128
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2009.128
Keywords
This article is cited by
-
Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions
Journal of Biosciences (2021)
-
Inhibiting the two-component system GraXRS with verteporfin to combat Staphylococcus aureus infections
Scientific Reports (2020)
-
New natural products to meet the antibiotic crisis: a personal journey
The Journal of Antibiotics (2019)
-
Screening for inhibitors of mutacin synthesis in Streptococcus mutans using fluorescent reporter strains
BMC Microbiology (2018)
-
Evidence of Cross-Regulation in Two Closely Related Pyruvate-Sensing Systems in Uropathogenic Escherichia coli
The Journal of Membrane Biology (2018)