Abstract
In search for new anti-varicella zoster virus (VZV) compounds with new mechanism of action, we applied a DNA hybridization assay (dot blot method) for screening. Using this method, we screened microbial products and found the polyether compound CP-44161 from the culture broth of an actinomycete strain. CP-44161 was previously reported as an anticoccidal agent, but there has been no claim of its antiviral activities. CP-44161 showed strong anti-VZV activity against pOka strain by plaque reduction assay. Moreover, CP-44161 showed lower cytotoxicity than other antiviral polyethers, such as monensin and nigericin. Its better safety margin and strong anti-VZV properties make it a good candidate for a new anti-VZV agent.
Similar content being viewed by others
Introduction
Varicella zoster virus (VZV) is a member of the Herpesviridae family that causes two distinct clinical syndromes. Primary infection is manifested as varicella (chickenpox). After the primary VZV infection, latent infection is established in the sensory nerve ganglia. Reactivation of latent VZV results in a localized eruption known as herpes zoster (shingles). Herpes zoster develops in approximately 30% of people over a lifetime. The risk of disease increases with age, and also frequently occurs in immunocompromised patients, especially those with human immunodeficiency virus (HIV) infection. Complications of herpes zoster in immunocompetent hosts include postherpetic neuralgia (PHN), encephalitis, myelitis, cranial nerve palsies and peripheral nerve palsies. PHN, a persistent pain syndrome occurring after the resolution of the zoster rash, is the most challenging complication.1
Standard antiviral drugs currently used in the treatment of VZV infections include acyclovir (ACV), valaciclovir (VACV, the oral prodrug of ACV), famciclovir (the oral prodrug of penciclovir) and vidarabine (Ara-A).2 A live attenuated VZV vaccine was originally developed as the ‘chickenpox vaccine’ to prevent varicella. Recently, a new VZV vaccine was developed for protection against herpes zoster.1
Despite a number of recent therapeutic advancements, there remains an urgent need to develop a new class of therapy, especially novel anti-VZV agent with a strong potency against VZV and with a different mechanism of action from nucleoside analogs, because current anti-VZV drugs exhibit lower potency against VZV than herpes simplex virus (HSV). In addition, they had some problems, such as cross-resistance and mutagenicity, which are inevitable consequences of nucleoside analogs.
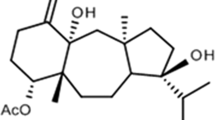
To discover a new candidate as an anti-VZV agent, we applied VZV DNA dot blot hybridization assay3 for screening anti-VZV compounds. Using this system, we screened anti-VZV agent among 34 000 microbial fermentation samples and found AS1720807 as a new anti-VZV compound. AS1720807 was the same as CP-44161, a polyether compound that was first reported as an anticoccidal compound from Dactylosporangium salmoneum FD 25647 (=IFO 14103) (Figure 2; Celmer et al.4 and Tone et al.5).
In this paper, we describe the strong anti-VZV activities of CP-44161 in vitro in comparison to existing anti-VZV agents and other polyethers that were reported to have antiviral activities.
Materials and methods
Cells and virus
Human malignant melanoma cell line (MeWo) was obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). Cells were grown and maintained in Eagle's minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, 0.075% NaHCO3 and 100 U ml−1 penicillin and 100 μg ml−1 streptomycin sulfate. Cells were grown at 37 °C in an atmosphere of 5% CO2.
VZV pOka strain and clinically isolated VZV YS strain6 were kindly provided by Dr T Suzutani (Fukushima Medical University). Cell-associated virus pools of VZV were produced by passing the virus for 48 h in MeWo cells, then trypsinizing the cells and resuspending the cells in the media containing 10% DMSO before freezing at −80 °C. Quantification of viral titers of stocks by the plaque assay and viral replication studies of VZV were performed in MeWo cells.
Reagents and antiviral compounds
Adenine 9-β-D-arabinofuranoside (Ara-A), monensin, nigericin, DMSO and 3-(4,5-dimethylthiazol-2-yl)-2,5,-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO, USA). ACV was purchased from GlaxoSmithKline (Tokyo, Japan). Bromovinyl arauracil (BV-araU) was kindly provided by Yamasa Corporation (Chiba, Japan). All compounds were dissolved in DMSO and then diluted with cell culture medium to yield 0.1% DMSO.
Screening method for anti-VZV compound
To quantify VZV DNA synthesis directly, we modified the VZV-DNA dot blot hybridization procedure described previously.3 MeWo cells were grown to confluence in F-225 plastic bottles and then infected with the VZV pOka strain (1.8 × 104 plaque forming units (PFU) per flask). After 8h incubation, cells were trypsinized and resuspended in the 96-well micro-titer plates. After 1h incubation, media in the presence or absence of various concentrations of the test compounds were added. After 36h incubation, individual wells were harvested by removing the cell culture medium and replacing it with a buffer consisting of 0.125 mg ml−1 RNase A, 0.5 mg ml−1 proteinase K, 0.05% SDS and 10 mM Tris-HCl (pH 7.5). After 3h incubation at 50 °C, 10 × SSC (1.5 M sodium chloride and 0.15 M sodium citrate) and 0.3 M NaOH were added and then neutralized by 1.0 M ammonium acetate. The samples were transferred to a nylon membrane (Roche Diagnostics, Tokyo, Japan) with a Bio-Dot SF Microfiltration Apparatus (Bio-Rad Laboratories, Tokyo, Japan). Filters were hybridized with VZV thymidine kinase (TK) DNA probe labeled by the digoxigenin (DIG) DNA labeling kit (Roche Diagnostics). Hybridization procedure was performed according to the manufacturer's protocol. DIG-labeled VZV DNA was detected by DIG Luminescent Detection kit (Roche Diagnostics) and the intensity of the spots was quantified by image analysis.
Plaque reduction assay
The antiviral effects of compounds against VZV were determined by plaque reduction assay. MeWo cells were grown to confluence in 24-well tissue culture plates and then infected with the VZV pOka strain or YS strain (40 PFU per well). After 1h incubation, media containing various concentrations of the compounds or 0.1% DMSO were added. After 3 days, the cells were stained with 1.0% crystal violet in 70% EtOH, and the number of viral plaques was counted.
Cytotoxicity assay
The cytotoxicity of compounds against growing host MeWo cells (2 × 104 cells per well in 96-well tissue culture plates) was determined with the MTT assay as the procedure described previously.7
Results
Screening for anti-VZV compound by DNA dot blot assay
Dot blot method has been shown to be a very sensitive and efficient method to detect VZV DNA from clinical specimens.3 However, there has been no report of isolating anti-VZV compound using this method. We applied the non-isotopic detection system by using the DIG-labeled VZV TK probe in this method (Figure 1) and simplified the process of DNA purification for facilitating the screening procedure.
Dot blot screening for anti-VZV compounds. VZV DNA levels in the presence or absence of AS1720807 (lanes 1A–5A, top dose 100 ng ml−1 with doubling dilution) were determined by the dot blot method. VZV-infected cells with BV-araU (lanes 1B–5B, top dose 1 ng ml−1 with doubling dilution) and ACV (lanes 1C–5C, top dose 1000 ng ml−1 with doubling dilution). Controls were performed for uninfected cells (7A), VZV-infected cells without drug treatment (lanes 6A–6C). Lane 7B represents 50% inhibition and lane 7C represents 75% inhibition control.
Next, to evaluate this method as a tool for screening new anti-VZV compounds, we compared the sensitivity of this method for anti-VZV drug to a conventional plaque reduction assay. As shown in Table 1, this method was as sensitive as the plaque assay; however, it could be completed in 36 h, which means that it could halve the assay period compared with the plaque assay. Moreover, its short assay period and direct detection of viral DNA gave us another benefit of drug screening. We found that this method reduced the assay noise of coexisting materials in microbial fermentation samples, such as inhibitors of viral adsorption and cytotoxic compounds.
Then we applied this method for screening anti-VZV compounds from microbial products. Among 34 000 microbial fermentation samples, we found a sample, which was an extract of fermentation broth of actinomycete strain no. 642788, that showed very strong anti-VZV activity. Using bioassay-guided preparation, we isolated the active substance as a powder and named it AS1720807.
Fermentation and isolation of AS1720807 (CP-44161)
Strain no. 642788, a producer strain of AS1720807, is an actinomycete strain that was isolated from soil sample collected in Malaysia. Strain no. 642788 was identified as Dactylosporangium sp. by morphological characteristics and phylogenic analysis of 16S rRNA gene sequence. The homology value between 642788 and IFO 14103 (D86938) was 99.0% (1436/1451) (data not shown).
A slant culture of strain was inoculated into a 225-ml Erlenmeyer flask containing 60 ml of a seed medium composed of soluble starch 1.0%, yeast extract 0.4%, gellan gum 0.5%, agar 0.1%, calcium chloride 0.0004%, pH 7.0 and cultured for 5 days at 30 °C. The seed culture (2.4 ml) was then inoculated into 500-ml Erlenmeyer flasks containing 120 ml of the production medium composed of soluble starch 2.0%, yeast extract 0.4%, allophane 1.0%, pH 7.0 and cultured for 5 days at 30 °C.
The broth was extracted with equal volume of Me2CO. The extract was applied on a DIAION HP-20 column and eluted with Me2CO, and concentrated. The concentrate was applied on a MicroBead SilicaGel 5D column, and eluted with 3:1 (v/v) of n-hexane-EtOAc. The active fractions were applied on a Daiso-gel SP-120-40/60-C4 column and eluted with 65% CH3CN containing 5.0 mM sodium phosphate buffer, pH 7.5. The pooled fractions were extracted with diethyl ether and concentrated under reduced pressure to obtain AS1720807 as a white powder.
By physico-chemical analysis, the structure of AS1720807 was determined as CP-44161 (Figure 2; Celmer et al.4 and Tone et al.5), a polyether previously reported as an anticoccidal compound (data not shown).
The following biological experiments were conducted with AS1720807 as CP-44161.
Anti-VZV activities of CP-44161
Antiviral activities of CP-44161 against the VZV pOka strain and clinically isolated YS strain were determined by plaque reduction assay. CP-44161 inhibited VZV replication in a dose-dependent manner (Figure 3). As shown in Table 2, the results gave an EC50 (concentration producing 50% inhibition) value against the pOka and YS strains for CP-44161 of 0.0051 and 0.015 μg ml−1 compared with an EC50 value for ACV of 2.7 and 2.9 μg ml−1 and for Ara-A of 1.8 and 5.7 μg ml−1 at 72 h, respectively. The results showed that CP-44161 had strong anti-VZV activity compared with current anti-VZV agents, ACV and Ara-A.
Anti-VZV activity and cellular toxicity of CP-44161 and other polyethers
Higher concentration (up to 1.0 μg ml−1) of CP-44161 was necessary to exhibit cytotoxicity in host MeWo cells. As CP-44161 was a polyether, we also compared anti-VZV activity and cytotoxicity with other polyether compounds, nigericin and monensin, which were reported to have anti-HSV activities.8, 9, 10 As shown in Table 3, nigericin and monensin showed strong anti-VZV activities, but they also exhibited strong cytotoxicity against host MeWo cells. As shown in Table 3, CP-44161 had a relatively higher selectivity index (ratio of 50% cytotoxic concentration to 50% inhibitory concentration against VZV replication) compared with other polyethers.
Discussion
The dot blot hybridization has been used to help diagnose several viral infections,11, 12 Seidlin et al.3 also developed this method for the detection of VZV DNA from clinical specimens. Dot blot method has been shown to be a very sensitive and efficient method to detect VZV replication; however, there has been no report of its application in drug screening for antiviral compounds. First, we introduced the non-isotopic detection system into dot blot method, and compared this method with a conventional plaque reduction assay in terms of its usefulness for drug screening and found some advantages; for example, short assay period and reduction of assay noise. Short assay period reduced the noise of coexisting cytotoxic compounds, and direct detection of viral DNA excluded pseudo-positive results that were sometimes observed in the plaque reduction assay.
By using this method to screen anti-VZV compounds, we found and isolated AS1720807 (CP-44161) from one of the microbial fermentation samples produced by Dactylosporangium sp. no. 642788. CP-44161 was previously reported as an anticoccidal agent, but there has been no claim of its antiviral activities. Next, we demonstrated that CP-44161 exhibited anti-VZV activity by the plaque reduction assay. CP-44161 inhibited viral replication in vitro in a dose-dependent manner. CP-44161 had more potent anti-VZV activities than clinically used agent, against not only laboratory strain but also clinical isolate.
As is well known, antibacterial mechanism of polyether antibiotics against Gram-positive bacteria is based on their inhibitory effect on the biological membrane ion transport.13 For some virus (for example, influenza virus), the low pH environment of the endosome triggers fusion of the virion envelope with cellular membranes.14, 15 Nakamura et al.16 showed the inhibitory effects of polyethers on HIV replication. Regarding the anti-herpes virus activities, nigericin and its derivative nigericinol, which have potassium ionophore activities, were reported to have anti-HSV-1 and 2 activities in vitro.8 Another polyether monensin, which has a sodium ionophore activity, inhibited HSV-1 (ref. 9,10) and human cytomegalovirus replication17 in vitro. Pleiotropic antiviral actions of monensin were reported. Johnson et al.9 and Ghosh-Choudhury et al.18 showed that monensin blocked late stages in the post-translational processing of the viral glycoproteins of HSV-1 and HSV-2.
In summary, we established an efficient in vitro screening system using DNA dot blot assay for anti-VZV compounds from crude microbial fermentation samples. Using this system, we isolated CP-44161, which had strong anti-VZV activity with lower cytotoxicity than other polyethers. Other polyethers, nigericin and monensin, also exhibited antiviral activities against VZV, but they also exhibited strong cytotoxicity against host cells. Nakamura et al.16 estimated the relative cytotoxicities of the polyethers against host cells of HIV. They showed that nigericin and lasalocid had a higher selectivity index than monensin. The results presented here were considered reasonable because the structure of CP-44161 was similar to lasalocid.
The mechanism by which CP-44161 inhibits VZV replication and the reason of its lower cytotoxicity are currently unknown. Lower cytotoxicity and strong anti-VZV properties of CP-44161 make it a good candidate for a new anti-VZV agent. Further experimental studies concerning its antiviral activity in vivo and the mode of action are under investigation.
References
Kimberlin, D. W. & Whitley, R. J. Varicella-zoster vaccine for the prevention of herpes zoster. N. Engl. J. Med. 356, 1338–1343 (2007).
De Clercq, E. Recent highlights in the development of new antiviral drugs. Curr. Opin. Microbiol. 8, 552–560 (2005).
Seidlin, M. et al. Detection of varicella-zoster virus by dot-blot hybridization using a molecularly cloned viral DNA probe. J. Med. Virol. 13, 53–61 (1984).
Celmer, W. D. et al. Polycyclic ether antibiotic produced by new species of Dactylosporangium, in United States Patent 4081532. Pfizer Inc., New York, NY, USA (1976).
Tone, J. et al. Abstract, 18th ICACC Meeting, Atlanta, Georgia (1978).
Sakuma, T. Strains of varicella-zoster virus resistant to 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Antimicrob. Agents Chemother. 25, 742–746 (1984).
Denizot, F. & Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 89, 271–277 (1986).
Grabley, S. et al. Secondary metabolites by chemical screening 17. Nigericinol derivatives: synthesis, biological activities, and modeling studies. J. Med. Chem. 35, 939–944 (1992).
Johnson, D. C. & Spear, P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43, 1102–1112 (1982).
Kousoulas, K. G., Bzik, D. J. & Person, S. Effect of the ionophore monensin on herpes simplex virus type 1-induced cell fusion, glycoprotein synthesis, and virion infectivity. Intervirology 20, 56–60 (1983).
Brandsma, J. & Miller, G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein–Barr viral DNA. Proc. Natl Acad. Sci. USA 77, 6851–6855 (1980).
Berninger, M. et al. An assay for the detection of the DNA genome of hepatitis B virus in serum. J. Med. Virol. 9, 57–68 (1982).
Harold, F. M. & Baarda, J. R. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J. Bacteriol. 95, 816–823 (1968).
Guinea, R. & Carrasco, L. Concanamycin A blocks influenza virus entry into cells under acidic conditions. FEBS Lett. 349, 327–330 (1994).
Daniels, P. U. & Edwardson, J. M. Intracellular processing and transport of influenza-virus envelope proteins in Madin–Darby canine kidney cells. Effects of the carboxylic ionophores monensin and nigericin. Biochem. J. 252, 693–700 (1988).
Nakamura, M. et al. Inhibitory effects of polyethers on human immunodeficiency virus replication. Antimicrob. Agents Chemother. 36, 492–494 (1992).
Kaiser, C. J. & Radsak, K. Inhibition by monensin of human cytomegalovirus DNA replication. Arch. Virol. 94, 229–245 (1987).
Ghosh-Choudhury, N., Graham, A. & Ghosh, H. P. Herpes simplex virus type 2 glycoprotein biogenesis: effect of monensin on glycoprotein maturation, intracellular transport and virus infectivity. J. Gen. Virol. 68, 1939–1949 (1987).
Acknowledgements
We thank Dr S Takase for NMR spectra measurement of CP-44161, Dr Neelam Shahab (SIRIM Rerhad, Malaysia) and Mr H Muramatsu for the culture of actinomycetes strain no. 642788, and Mr T Zenkoh, Dr K Sudo and Dr K Maki for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamagishi, Y., Ueno, C., Kato, A. et al. Discovery of anti-varicella zoster virus activity of polyether antibiotic CP-44161. J Antibiot 62, 89–93 (2009). https://doi.org/10.1038/ja.2008.19
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ja.2008.19
Keywords
This article is cited by
-
Anti-herpes virus activity of polyether antibiotic CP-44161 in vivo
The Journal of Antibiotics (2009)