Abstract
Proteorhodopsins (PR) are light-driven proton pumps widely distributed in bacterioplankton. Although they have been thoroughly studied for more than a decade, it is still unclear how the proton motive force (pmf) generated by PR is used in most organisms. Notably, very few PR-containing bacteria show growth enhancement in the light. It has been suggested that the presence of specific functions within a genome may define the different PR-driven light responses. Thus, comparing closely related organisms that respond differently to light is an ideal setup to identify the mechanisms involved in PR light-enhanced growth. Here, we analyzed the transcriptomes of three PR-harboring Flavobacteria strains of the genus Dokdonia: Dokdonia donghaensis DSW-1T, Dokdonia MED134 and Dokdonia PRO95, grown in identical seawater medium in light and darkness. Although only DSW-1T and MED134 showed light-enhanced growth, all strains expressed their PR genes at least 10 times more in the light compared with dark. According to their genomes, DSW-1T and MED134 are vitamin-B1 auxotrophs, and their vitamin-B1 TonB-dependent transporters (TBDT), accounted for 10–18% of all pmf-dependent transcripts. In contrast, the expression of vitamin-B1 TBDT was 10 times lower in the prototroph PRO95, whereas its vitamin-B1 synthesis genes were among the highest expressed. Our data suggest that light-enhanced growth in DSW-1T and MED134 derives from the use of PR-generated pmf to power the uptake of vitamin-B1, essential for central carbon metabolism, including the TCA cycle. Other pmf-generating mechanisms available in darkness are probably insufficient to power transport of enough vitamin-B1 to support maximum growth of these organisms.
Similar content being viewed by others
Introduction
The ocean’s surface is the largest sun-lit environment on Earth. Most of the global biological activity such as photosynthesis and respiration takes place in the photic zone of the ocean, having a major impact on the overall carbon cycle. It has been recognized for many decades that marine organisms capture energy from sunlight via well-known chlorophyll-based photosynthesis and to a much lesser extent via bacteriochlorophyll-a (Shiba et al., 1979). However, in recent years, it has been found that a third pigment type, proteorhodopsins (PR), may also be important. PRs are light-driven proton pumps that can be used for energy (ATP) generation (Béjà et al., 2000; Martinez et al., 2007). They are widely distributed in all environments where sunlight can reach, particularly in aquatic settings. The photic zone of the ocean contains the largest pool of PR, with an average of 75% of all bacterioplankton cells carrying these genes (Sabehi et al., 2005; Rusch et al., 2007). Since the discovery of PRs in the year 2000, a considerable amount of effort has been dedicated to discern their abundance, diversity and biochemical functioning (Béjà et al., 2000; 2001; de la Torre et al., 2003; Sabehi et al., 2003; 2005; Martinez et al., 2007). However, although they have been perceived as relatively simple photosystems, the consequences of their activity on cell physiology at both the individual and community level are still poorly understood.
Studies of PR gene expression in microbial communities in situ as well as in light-dark incubations have so far produced varied results, possibly indicative of multiple distinct utilization strategies at work (Fuhrman et al., 2008). Physiological responses at the organism level such as light-enhanced growth, increased survival as well as substrate uptake, have been observed in phylogenetically diverse groups of PR-containing bacteria in culture. One example is Candidatus Pelagibacter ubique (Ca. P. ubique) HTCC1062, for which a greater survival capacity and ATP-dependent substrate uptake in the light have been reported (Steindler et al., 2011). Also, two closely related marine Vibrio strains (AND4 and BAA-1116) have shown better survival to starvation or respiratory stress in the light (Gómez-Consarnau et al., 2010; Wang et al., 2012). However, a light-stimulated growth response could not be detected in other cultured bacteria like the gammaproteobacterium HTCC2207 in the SAR92 clade (Stingl et al., 2007). To gain a complete understanding of the ecological impact of PR photoheterotrophy, the growth responses of PR-producing bacteria need to be resolved.
Flavobacteriia is the bacterioplankton class on which most physiological studies of PR have been conducted and most biological responses to light have been detected. For example, Polaribacter sp. MED152 has shown greater bicarbonate uptake in the light (González et al., 2008) and the growth of Psychroflexus torquis ATCC 700755T was improved under salinity stress upon illumination (Feng et al., 2013). Dokdonia MED134 was the first bacterium shown to divide faster in the light compared to the dark under low organic carbon concentrations (Gómez-Consarnau et al., 2007). Subsequent studies on the same strain have shown that growth stimulation by PR in the light is linked to changes in central metabolism (Palovaara et al., 2014) and/or transcriptional changes associated with Na+ pumping (Kimura et al., 2011). Recently, another flavobacterium, Nonlabens marinus S1-08, containing three different rhodopsin types (H+, Na+ and Cl− pumps) has been described. Similarly to MED134, this bacterium grows better in the light compared with dark in a low organic matter medium (Yoshizawa et al., 2014). In contrast to these two Dokdonia (S1-08 and MED134), another strain of the same genus, PRO95, has not yet shown enhanced growth or increased PR gene expression in the light compared to the dark (Riedel et al., 2010). PRO95 also has more than one rhodopsin type and, according to sequence similarities, one functions to pump H+ and the other one to pump Na+ ions (Yoshizawa et al., 2014, Bertsova et al., 2015). Together, the studies of PR in cultured bacteria reflect a diversity of physiological responses that still cannot be predicted solely based on whole-genome sequence information, and therefore a combination of different research approaches are required to elucidate the specific mechanisms of PR photoheterotrophy.
Proton gradients and ATP generated by PR can potentially affect the global ocean carbon budgets in two ways: (i) they could decrease carbon respiration rates, if less dissolved organic carbon is respired and preferably used for anabolic processes, as shown for aerobic anoxygenic phototrophs (Holert et al., 2011; Tomasch et al., 2011), or (ii) they could enhance the uptake of solutes that could subsequently be respired via PR-powered ion gradients. Both of these mechanisms have been shown for Ca. P. ubique HTCC1062, a member of the abundant SAR11 clade. Respiration rates of SAR11 bacteria decreased under starvation in the light (Steindler et al., 2011). However, when these bacteria were provided with taurine, ATP-dependent transport of the substrate was observed, suggesting that this resource became available for respiration under light conditions. Indeed, the intracellular transport of most substrates depends on either ATP (ABC transporters) or H+ gradient pumps (TonB-dependent transporter (TBDT), H+ antiporters) (Wong and Buckley, 1989; Bradbeer, 1993), suggesting that PR could have an important role in the transport efficiency of dissolved nutrients and growth factors such as B-vitamins.
The objectives of the study were to evaluate: (i) whether the observed specific PR-driven responses to light are due to the presence or absence of specific functions within a particular bacterial genome, as recently hypothesized (González et al., 2011; Riedel et al., 2013) and (ii) whether the energy generated by PR could be used for the uptake of exogenic organic molecules (for example, B-vitamins). To accomplish this, we compared three bacteria in the genus Dokdonia (MED134 from the Mediterranean Sea, PRO95 from the North Sea, and DSW-1T from the East Sea of Korea), which contain PR genes and respond differently to light exposure. Nucleotide identity among 16S rRNA genes of the three marine Dokdonia was 99.5%. However, PRO95 belongs to a separate species, as the estimated DNA-DNA hybridization value was 25% with respect to DSW-1T and 63% with MED134, lower than the discriminatory value of 70% for strains within the same species (Auch et al., 2010). Our genome analysis revealed that although the three Dokdonia strains have a very similar gene content (Supplementary Table S1), only PRO95 has the de novo pathway to synthesize vitamin-B1, an organic cofactor required for the primary metabolism of all living organisms (for example, TCA cycle of respiration) (Voet and Voet, 2004). Transcriptome comparisons showed that the expression of PR genes was higher in the light compared with dark in the three Dokdonia. However, the light-enhanced growth was only observed in DSW-1T and MED134. Furthermore, the high expression of vitamin-B1 TBDT in the two auxotrophic Dokdonia (DSW-1T and MED134) and the potential powering of those transporters by PR functioning as a H+ pump could explain the observed growth response to light. This inferred mechanism for light-enhanced growth has not previously been described.
Materials and methods
Genome sequencing of DSW-1T
DSW-1T was obtained from the culture collection DMSZ (DSM-17200). It was grown on ZoBell agar plates until colonies were identified and then inoculated in 40 ml ZoBell liquid medium for 72 h at room temperature. Genomic DNA was extracted from 15 ml pellets using DNeasy kit (Qiagen, Hilden, Germany) and then sheared using Bioruptor NGS (Diagenode, Denville, NJ, USA). The genomic library was prepared using a liquid handling Apollo 324 robot (InteGenX, Pleasanton, CA, USA) and the KAPA HTP Library Preparation Kit for Illumina Platforms (Kapa Biosystems, Wilmington, MA, USA). Ligation products were purified and subjected to a final PCR amplification (10 cycles). The amplified library was assessed by Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA) and real-time quantitative PCR (Kapa Biosystems) and subsequently sequenced on Illumina MiSeq for paired read of 250 bases (Illumina, San Diego, CA, USA). Sequence data were quality-trimmed to Phred 25 and read pairs merged using a Conveyor workflow (Linke et al., 2011); non-overlapping read pairs were discarded. Owing to high coverage (~470 ×), random subsamples of the merged read pairs were individually assembled using the Roche GS de Novo Assembler and SPAdes version 3.0 software packages (Bankevich et al., 2012). The assemblies were merged by re-sampling longer reads from the obtained contigs and performing a final assembly using the Roche GS de Novo Assembler (Roche Applied Science, Branford, CT, USA). The genome was finally assembled into a total of 20 contigs.
This Whole Genome Shotgun project has been deposited at DDBJ/EMBL/GenBank under the accession JSAQ00000000. The version described in this paper is version JSAQ01000000.
Growth conditions
Strains DSW-1T, MED134 and PRO95 were grown in the same conditions in artificial seawater using sea salts (Sigma-Aldrich, St Louis, MO, USA) at 35 practical salinity units amended with 0.39 mM DOC from ZoBell medium, 2.1 μM NH4Cl and 0.3 μM Na2HPO3 as in Gómez-Consarnau et al. (2007). No additional vitamins were added to the medium. The 1.9-l cultures were kept in 2-l polycarbonate bottles in a temperature-controlled room at 18 °C and exposed to light or darkness in duplicates. Light treatments were incubated under continuous light (130 μmol photons m−2 s−1) using an artificial white light source. Dark bottles were covered with aluminum foil and a black plastic layer. Bacterial growth was monitored using cell counts every 24 h. Two milliliter samples were fixed with 10% formalin (4% formaldehyde), stained with acridine orange (Hobbie et al., 1977), filtered through pre-blackened filters and counted with epifluorescence microscopy.
Vitamin-B1 quantification in the culture medium
The concentration of vitamin-B1 in the axenic growth medium was quantified using HPLC-MS (Sañudo-Wilhelmy et al., 2012) after autoclaving (t0) and after 5 days incubation in the light (t5; sampling time for transcriptomic samples) in duplicate bottles, using triple injections per sample.
RNA sampling, processing and sequencing
After 5 days of growth, bacterial biomass was collected by filtering 1 l of culture onto 0.22-μm pore size, 47-mm diameter Durapore (Millipore, Billerica, MA, USA) filters using vacuum filtration. Immediately after filtration, filters were incubated for 1 min with 2 ml RNA-protect and stored dry at −80 °C. RNA was extracted using the RNAeasy Mini kit (Qiagen), with two DNase digestions as in Riedel et al. (2013). The standard protocol for library construction began with 1 μg of total RNA. RNA was treated with the Epicentre Ribo-Zero ribodepletion kit for Gram-negative bacteria (Epicenter, Madison, WI, USA). Following ribodepletion, the entire sample was introduced into the Illumina TruSeq mRNA v2 protocol at the elute-prime-fragmentation step for cDNA generation using 12 PCR cycles. Libraries were visualized by Bioanalyzer (Agilent) and quantified for pooling and sequencing using Kapa Biosystems qPCR quantification kit.
For sequencing, libraries were diluted to 16 pM and then applied to a V3 flowcell using the Illumina cBot clonal amplification device. Libraries were sequenced on the Hi-Seq 2000 using HSCS v 1.5.15.1 obtaining more than 30 million reads per sample. The sequences were then quality-trimmed using FASTX-Toolkit (http://hannonlab.cshl.edu/fastx_toolkit), which discarded 2–3% of the reads. From these, 99% of the trimmed sequences mapped to the genome of the three strains using the program segemehl (Hoffmann et al., 2009). Read abundance was extracted from resulting SAM mapping files with SAMtools version 0.1.19 (Li et al., 2009) and coverage per base was calculated from BAM files. The read mapping accuracy and the levels of expression of the key genes used in this study (for example, PR, TBDT, vitamin-B1 synthesis) as well as for other highly expressed genes (for example, ribosomal proteins) were visually inspected with the programs Rockhopper (Tjaden, 2015) and Integrative Genomics Viewer (Thorvaldsdottir et al., 2013). In addition, plots based on aligned sequence reads per base were made with Python scripts for comparison. Read counts per gene were obtained from SAM alignment files with HTSeq software. To identify the differentially expressed genes, we used the DESeq R/Bioconductor package (Anders and Huber, 2010). The DESeq package normalizes for sequencing depth and it is based on a negative binomial distribution model of read counts (Robinson and Smyth, 2007; Anders and Huber, 2010).
The genomes of DSW-1T and PRO95 were submitted to RAST for automatic annotation (Aziz et al., 2008). The annotation of genes in DSW-1T and PRO95 was then matched to the manual annotation of MED134 (González et al., 2011) when the putative gene product was shared. That is, when reciprocal Best Hits based on BLASTP against the peptides of MED134 had a minimum percent identity=35, maximum evalue=1 and a minimum percent coverage=50. The genes of DWS-1T and PRO95 that had no homologs in MED134 were annotated de novo as described in the study by González et al. (2011). The assignment of the individual peptides to functional categories as KEGG Orthology terms (KO; Kyoto Encyclopedia of Genes and Genomes (KEGG); Kanehisa et al., 2007) was based on homology search by BLASTP against the KEGG database of peptides with a minimum percent identity of 35, minimum coverage of 60 and minimum bitscore of 50. Transcriptome sequences were uploaded to the European Nucleotide Archive under the Study accession PRJEB10816.
Results and Discussion
Genomic comparison among the three Dokdonia strains
We investigated the photo-physiology and gene expression of three closely related strains in the genus Dokdonia. Nucleotide identity between 16S rRNA genes of the three strains was 99.5%. On the basis of genome-to-genome distance calculator GGDC analysis (Auch et al., 2010), PRO95 belong to separate species as the estimated DNA-DNA hybridization value (only 25% identity with DSW-1T and 63% with MED134). This result was confirmed with an average nucleotide identity analysis (95.93%), which puts them on the lower limit range for strains within the same species (95–96%) (Supplementary Table S1) (Goris et al., 2007). We refer to the strain designation hereafter because only the nomenclature of DSW-1T has been formally described. The genomes of MED134 and PRO95 had been sequenced and described earlier (González et al., 2011; Riedel et al., 2013). In this study, we have sequenced the genome of the type strain, D. donghaensis DSW-1T (Yoon et al., 2005), allowing a broader comparison within this genus. The new genome is 3 223 976 nucleotides in length, similar to MED134 (3 302 550 nucleotides) and PRO95 (3 303 993 nucleotides). On the basis of the gene content of the three strains, the Dokdonia core genome contained 2389 protein-coding genes (77–82% of the genomes) (Figure 1, Supplementary Table S1). Although the overall genomic features among the three strains were very similar (Table 1), DSW-1T and MED134 shared a larger number of protein-coding genes with each other (87.5% of DSW-1T genome and 89.4% of MED134 genome) than with PRO95 (79%) (Figure 1, Supplementary Table S1), which is consistent with their phylogenetic proximity.
Venn diagram showing the genes shared by the Dokdonia strains DSW-1T, MED134 and PRO95. Numbers indicate the genes that were unique for each organism as well as the ones shared by two or the three strains. The diagrams were made using the program eulerAPE (http://www.eulerdiagrams.org/eulerAPE/).
The three Dokdonia genomes contain rhodopsin genes, which makes them photoheterotrophs. While MED134 and DSW-1T have one copy of the rhodopsin gene, PRO95 contains two different rhodopsin genes: a H+ rhodopsin (PR) and a Na+ rhodopsin (NaR), based on sequence comparison (Yoshizawa et al., 2014; Bertsova et al., 2015). This would a priori suggest that PRO95 has a greater phototrophic capacity compared with the other two strains. However, previous light incubation experiments using this organism did not detect a light-enhanced growth response (Riedel et al., 2010; 2013). The PR peptide of DSW-1T differs from that of MED134 in only one amino acid while the identity with that of PRO95 is 74%. These differences in PR sequence might indicate that PRO95’s PR is the result of lateral gene transfer, as the presence of genomic islands has frequently been found in Flavobacteria (González et al., 2011; Fernández-Gómez et al., 2013).
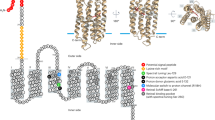
Another important feature that differentiates these organisms is that the PRO95 genome contains the de novo pathway to synthesize vitamin-B1 (thiE, thiF, thiD, thiH, thiG, thiC, thiS, thiL) (Figure 2) (Jurgenson et al., 2009), whereas MED134 and DSW-1T do not. Figure 3 illustrates the presence/absence of the enzymes required at each step of the synthesis (or uptake) of vitamin-B1 in the three Dokdonia strains. DSW-1T and MED134 lack the entire set of genes for the synthesis of vitamin-B1 except for dxs, which is also used in other metabolic pathways (Lois et al., 1998). The three strains have a thiamin pyrophosphate riboswitch next to a TBDT receptor (MED134_10161 and homologs in the other two strains). This may indicate that the organisms could sense the intracellular presence of B1-vitamin and repress the gene expression for the specific TBDT when the intracellular concentration of the vitamins is sufficiently high (Winkler and Breaker, 2005). PRO95 has an additional thiamin pyrophosphate riboswitch next to thiC, the first gene in an eight-gene cluster encoding thiamin synthesis genes (Figure 2), indicating that this organism could also accurately control the synthesis of this vitamin. Vitamin-B1 is composed of two moieties: the HMP (4-amino-5-hydroxymethyl-2-methylpyrimidine) and THZ (4-methyl-5-(2-hydroxyethyl)-thiazole). In addition to the vitamin-B1 uptake transporters, we identified the ABC transporter genes for the HMP moiety in MED134 (MED134_00580) and DSW-1T but not for the transport of THZ (Figure 3).
Genomic region where the vitamin-B1 synthesis operon of PRO95 is located. Green arrow shows the thiamin pyrophosphate riboswitch and in red are the vitamin-B1-synthesis genes. The genomic area around this operon is shared by the three Dokdonia strains although the synthesis genes are only present in PRO95.
Diagram of the de novo synthesis pathway of vitamin-B1. Green, orange and red boxes indicate the presence of the specific genes in DSW-1T, MED134 and PRO95, respectively. The red dashed lines indicate transport functions. Question marks indicate that those genes have not been found but would be expected to be present in the genomes.
Growth responses of Dokdonia in light and dark
To compare their growth response to light exposure, the three Dokdonia were grown in an artificial seawater medium. All strains grew in the dark and in the light, but with substantial differences in growth rates (Table 2, Supplementary Figure S1). Light-enhanced growth was higher in DSW-1T (3.3-fold higher cell yields in the light compared to the dark) than in MED134 (2.1-fold higher yields under illumination), while PRO95 showed no significant differences in growth between light and dark treatments. However, cell yields were significantly different; PRO95 reached the highest biomass of all (7.76 × 106 cells per ml), followed by the light treatments of MED134 (5.76 × 106 cells per ml) and DSW-1T (0.9 × 106 cells per ml). These different growth responses observed in the Dokdonia strains were evident despite the high variability observed in the growth measurements of DSW-1T and PRO95 (Table 2, Supplementary Figure S1). Light-mediated destruction of vitamin-B1 was tested by measuring its concentration after 5 days in the light in sterile medium. We could not find evidence for a light effect on thiamin concentrations at around 100 pM.
Overall regulation of gene expression under light and dark conditions
We used a transcriptomic analysis to infer the mechanisms used by each Dokdonia strain to adjust its metabolism to light availability. To establish a threshold, we considered that differential gene expression was significant when the light vs dark transcript linear fold change was greater than 2 (higher in the light) or lower than 0.5 (higher in the dark) and with an adjusted P-value (P-adj) lower than 0.05. Consistent with the observed differences in cell yields (Table 2), the light-dark differential expression in these organisms was strikingly different despite their relatedness (Figures 4 and 5). DSW-1T was the organism with the highest number of differentially expressed genes (617; 194 higher in the light and 423 higher in the dark), followed by MED134 (170; 144 in the higher light and 26 higher in the dark) and PRO95 (20; 16 higher in the light and 4 higher in the dark) (Figure 5).
Normalized mean mRNA read counts of the Dokdonia transcriptomes. (a) MED134, (b) DSW-1T and (c) PRO95. In red are the reads that are significantly higher in light or dark (P-adj<0.05) regardless of the light/dark fold change value. Horizontal black lines denote the linear fold change levels of 2 and 0.5. Circled green and dark blue dots denote the PR and NaR transcript reads, respectively. Transcript sequence data were normalized by sequencing depth as described in the DESeq package (see Materials and methods).
(a) Total transcripts that were significantly higher in light (in yellow) or dark (in black) in DSW-1T, MED134 and PRO95. Significantly higher expression was considered when the linear fold change in light vs dark transcripts was larger than 2 or lower than 0.5 and the P-adjusted values were<0.05. Venn diagrams show the number of transcripts that were significantly higher in light (b) or dark (c) for each strain exclusively, as well as the number of transcripts shared by two or the three organisms in the two treatments. The Venn diagrams were made using the program eulerAPE (http://www.eulerdiagrams.org/eulerAPE/).
Expression of rhodopsin under light-dark conditions
The expression of PR (and NaR of PRO95) was above the average gene expression for the three Dokdonia strains and significantly higher in the light (Figure 4). The only transcripts that were significantly higher in the light for the three strains were, in addition to the PR, a PAS domain sensor protein (MED134_10396 and homologs in DSW-1T and PRO95) and a hypothetical protein (MED134_04254) (Supplementary Table S3A). PAS domains can respond to multiple stimuli like cellular energy levels, oxygen levels, redox potential and light (Taylor and Zhulin, 1999). Although this PAS domain sensor gene is not in the region nearby the PR gene, its presence and high expression in the light observed in the three strains suggest that this is an important part of the light-dependent regulation. Notably, PR genes were also the transcripts with highest fold change in the light compared with the dark, being 14 in DSW-1T and 26.6 in MED134. In PRO95, two adhesion proteins (that closest match to MED134_13296 and MED134_13296, 34 × and 32 ×) showed the highest fold change followed by the PR (fold change of 20) (Figure 4, Supplementary Table S3). Thus, PRO95 had a higher expression of the rhodopsin genes in the light although it grew equally well in light or darkness, indicating that the differential expression of PR genes was not directly related to the light-enhanced growth and that other elements of their metabolism need to be considered.
Proton motive force (pmf)-dependent processes
Out of all the processes that require a pmf for their activity (that is, membrane transport and motility) (Saier, 2000; Jarrel and McBride, 2008), the highest numbers of transcript reads in the three strains were for TBDT genes followed by gliding motility (Figure 6a). The TBDT family contains integral membrane proteins that use proton gradients, like the ones produced by PR, to transport molecules of different molecular weight across the outer bacterial membrane (reviewed by Tang et al., 2012). Their high expression supports previous results on the lifestyle of Flavobacteria as degraders of high molecular weight compounds (Cottrell and Kirchman, 2000; Cottrell et al., 2005; Teeling et al., 2012). Most of the known TBDT have not been characterized yet, so the specific substrate they transport is currently unknown. However, analysis of operons suggests that they might be involved in the uptake of polysaccharides, peptides, heme groups and/or vitamins. In our study, we observed two susC TBDT of particularly high expression in MED134 and DSW-1T (MED134_12381 and MED134_05219) (Supplementary Table S2), which are possibly involved in the uptake of high molecular weight compounds such as proteins or complex carbohydrates (González et al., 2011; Tang et al., 2012).
(a) Gene expression levels of pmf-dependent processes. Gray shading denotes the dark treatment transcriptome samples. (b) Average number of transcripts within light and dark treatments of vitamin-B1 (thiamin pyrophosphate)-dependent enzymes as well as vitamin-B1 transport and synthesis in DSW-1T, MED134 and PRO95. mRNA sequence data were normalized by sequencing depth as described in the DESeq package (see Materials and methods).
Vitamin-B1 metabolism and PR phototrophy
PRO95 was the only Dokdonia strain among the three investigated here that contained the de novo pathway for vitamin-B1 synthesis (Figure 3). We detected the expression of at least one gene in the synthesis pathway of vitamin-B1, thiC, among the most highly expressed genes of PRO95 (Supplementary Table S2). Furthermore, the vitamin-B1-TBDT (MED134_10161 and homolog in DSW-1T) was the third most highly expressed TBDT gene in the vitamin-B1 auxotrophs MED134 and DSW-1T (Figure 6b, Supplementary Table S2), suggesting that vitamin-B1 was essential for them all. Indeed, the vitamin-B1 TBDT transcripts were about 10 times higher in the vitamin-B1 auxotrophs (DSW-1T and MED134) compared with the synthesizer PRO95 (Figure 6b). Vitamin-B1 transporter genes accounted for as much as 18% of all pmf-dependent processes. Our results are consistent with the transcriptome of MED134 reported by Kimura et al. (2011), where we found (reanalyzing their Supplementary Materials) that vitamin-B1 TBDT were also among the most highly expressed genes.
Although the vitamin-B1 requirements for heterotrophic bacteria have not been established, we suggest that the observed high expression of thiamin pyrophosphate riboswitch-regulated genes could be related to low vitamin-B1 intracellular levels and availability. This is because the riboswitches should repress gene expression when the vitamin concentration meets their intracellular requirements (Winkler and Breaker, 2005). We did not observe any significant changes in vitamin-B1 concentrations in the growth medium under our experimental conditions (t0=99.3 ±17.5 pM vs t5=81.4 ±11.2 pM; Mann–Whitney test, P=0.33). This suggests that the gene expression patterns observed in the Dokdonia strains were not due to a light-mediated destruction of the vitamin. The light stability of vitamin-B1 is consistent with the use of an artificial white light source in our experiments, as vitamin-B1 light degradation has been observed only under x-rays, gamma rays and UV irradiation (Gubler, 1984). Vitamin-B1 concentrations in the growth medium were within the threshold levels considered limiting for some phytoplankton species (Carlucci and Silbernagel, 1966; Tang et al., 2010; Paerl et al., 2015), suggesting that bacteria could have been vitamin-B1-deficient during our experiments.
Moreover, when our transcriptomics samples were grouped by growth phase (regardless of light or dark treatment and isolate) only six gene transcripts were higher than the average in the exponential phase (>10%) but lower in the stationary phase (<10%) (Supplementary Table S5). One of those genes encoded a ThiJ/PfpI family protein (MED134_04464), involved in vitamin-B1 metabolism, most likely in the transformation of thiamin into thiamin’s active form thiamin pyrophosphate (thiN), suggesting that vitamin-B1 is highly required during active growth. Of all the vitamin-B1-dependent genes of Dokdonia, the most highly expressed were involved in the TCA cycle (2-oxoglutarate dehydrogenase E1 component, sucA, and pyruvate dehydrogenase E2 component; MED134_07711 and MED134_12071), one of the main metabolic steps in the oxidation of carbohydrates, fats and proteins into CO2, fueling the production of ATP (Supplementary Table S4).
A recent study also used Dokdonia MED134 grown in yeast extract and peptone medium (YEP), and discussed the reactions of the TCA cycle that may be involved in the light-enhanced growth (Palovaara et al., 2014). They quantified the expression of a small set of genes (11), including only one vitamin- B1-dependent enzyme, 2-oxoglutarate dehydrogenase. In both, their and our study, this enzyme was not differentially expressed in the light or in the dark when growing in YEP medium. As this enzyme is central for cellular growth, and vitamin-B1 is its coenzyme, we hypothesize that vitamin-B1 auxotrophic Dokdonia did not grow well in the dark owing to vitamin deprivation.
Figure 7 illustrates the proposed scenarios where the vitamin, being either transported into the cell (DSW-1T and MED134) or synthesized de novo (PRO95), can have an impact on growth of Dokdonia. For the vitamin-B1 auxotrophs DSW-1T and MED134, vitamin-B1 transport could be substantially enhanced by the pmf generated by PR in the light. In the dark, even though PR and vitamin-B1 -TBDT might be present and abundant, the lack of PR pmf would lead to insufficient intracellular vitamin-B1 concentrations, and growth rates would be reduced to suboptimal operation of the TCA cycle and other vitamin-B1-dependent pathways. In contrast, the vitamin-B1 synthesizer (PRO95) would not depend on environmental concentrations or the transport of vitamin-B1, allowing the organism to continue maximum growth even in the dark (Figure 7).
Conclusions
The aim of this study was to try to understand the mechanisms by which Dokdonia (and perhaps other bacteria) that contain PR photosystems grow better in the light while others do not. In Dokdonia, vitamin-B1 metabolism and the use of specific vitamin-B1 TBDT seem to be relevant for the light-dependent growth response. In contrast to other substrates that can be utilized for growth, B-vitamins have extremely precise functions that cannot be replaced by any other molecule (Voet and Voet, 2004; Sañudo-Wilhelmy et al., 2014), thus leading to growth limitation when absent. It has recently been shown that large areas of the ocean are vitamin-depleted, suggesting that vitamin limitation is commonly found in situ (Sañudo-Wilhelmy et al., 2012). Moreover, all genome-sequenced members of the abundant marine SAR11 clade can synthesize only the THZ moiety of the vitamin-B1, and their growth can be significantly limited by the absence of the HMP moiety or thiamin itself (Carini et al., 2014). These results suggest that the PR light-mediated uptake of vitamin-B1 and its auxotrophy could be important in vitamin-B1-limited environments. Having the de novo pathway for vitamin-B1 synthesis, other microbes (for example, Dokdonia PRO95) would be unaffected by fluctuations in ambient vitamin-B1 availability, growing equally well in the light or in the dark. However, the auxotrophy and limitation by other vitamins or any other irreplaceable coenzymes would potentially lead to the situation observed in the strains Dokdonia DSW-1T and MED134 with respect to vitamin-B1.
Finally, we hypothesize that the light-enhanced growth seen in some Dokdonia strains is not caused through reduction of respiration under illumination, but to the possibility to sustain aerobic respiration (via the TCA cycle) when vitamin-B1 limitation is alleviated through PR activity. Future studies will need to address the effects of this hypothetical PR light-enhanced respiration in Dokdonia and other marine PR photoheterotrophs to further evaluate its impact on the carbon cycle.
Accession codes
References
Anders S, Huber W . (2010). Differential expression analysis for sequence count data. Genome Biol 11: R106.
Auch AF, Klenk H-P, Göker M . (2010). Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genomic Sci 2: 142–148.
Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA et al. (2008). The RAST Server: Rapid Annotations using Subsystems Technology. BMC Genomics 9: 75.
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19: 455–477.
Béjà O, Aravind L, Koonin EV, Suzuki MT, Hadd A, Nguyen LP et al. (2000). Bacterial rhodopsin: evidence for a new type of phototrophy in the sea. Science 289: 1902–1906.
Béjà O, Spudich EN, Spudich JL, Leclerc M, DeLong EF . (2001). Proteorhodopsin phototrophy in the ocean. Nature 411: 786–789.
Bertsova YV, Bogachev AV, Skulachev VP . (2015). Proteorhodopsin from Dokdonia sp. PRO95 is a light-driven Na+ -pump. Biochemistry (Moscow) 80: 449–454.
Bradbeer C . (1993). The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J Bacteriol 175: 3146–3150.
Carini P, Campbell EO, Morré J, Sañudo-Wilhelmy SA, Cameron Thrash J, Bennett SE et al. (2014). Discovery of a SAR11 growth requirement for thiamin's pyrimidine precursor and its distribution in the Sargasso Sea. ISME J 8: 1727–1738.
Carlucci AF, Silbernagel SB . (1966). Bioassay of seawater II. Methods for the determination of concentrations of dissolved vitamin B1 in seawater. Can J Microbiol 12: 1079–1089.
Cottrell MT, Kirchman DL . (2000). Natural assemblages of marine proteobacteria and members of the Cytophaga-Flavobacter cluster consuming low- and high-molecular-weight dissolved organic matter. Appl Environ Microbiol 66: 1692–1697.
Cottrell MT, Yu L, Kirchman DL . (2005). Sequence and expression analyses of cytophaga-like hydrolases in a Western Arctic metagenomic library and the Sargasso sea. Appl Environ Microbiol 71: 8506–8513.
de la Torre JR, Christianson LM, Béjà O, Suzuki MT, Karl DM, Heidelberg J et al. (2003). Proteorhodopsin genes are distributed among divergent marine bacterial taxa. Proc Natl Acad Sci USA 100: 12830–12835.
Feng S, Powell SM, Wilson R, Bowman JP . (2013). Light-stimulated growth of proteorhodopsin-bearing sea-ice psychrophile Psychroflexus torquis is salinity dependent. ISME J 7: 2206–2213.
Fernández-Gómez B, Richter M, Schüler M, Pinhassi JHJ, Acinas SG, González JM et al. (2013). Ecology of marine Bacteroidetes: a comparative genomics approach. ISME J 7: 1026–1037.
Fuhrman JA, Schwalbach MS, Stingl U . (2008). Proteorhodopsins: an array of physiological roles? Nat Rev Micro 6: 488–494.
Gómez-Consarnau L, Akram N, Lindell K, Pedersen A, Neutze R, Milton DL et al. (2010). Proteorhodopsin phototrophy promotes survival of marine bacteria during starvation. PLoS Biol 8: e1000358.
Gómez-Consarnau L, González JM, Coll-Llado M, Gourdon P, Pascher T, Neutze R et al. (2007). Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature 445: 210–213.
González JM, Fernández-Gómez B, Fernàndez-Guerra A, Gómez-Consarnau L, Sánchez O, Coll-Llado M et al. (2008). Genome analysis of the proteorhodopsin-containing marine bacterium Polaribacter sp. MED152 (Flavobacteria). Proc Natl Acad Sci USA 105: 8724–8729.
González JM, Pinhassi JHJ, Fernández-Gómez B, Coll-Lladó M, González-Velazquez M, Puigbo P et al. (2011). Genomics of the proteorhodopsin-containing marine flavobacterium Dokdonia sp. strain MED134. Appl Environ Microbiol 77: 8676–8686.
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM . (2007). DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57: 81–91.
Gubler CJ . (1984). Thiamin. In: Machlin LJ. (ed). Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects. Marcel Dekker, Inc: New York, NY, USA, pp 245–297.
Hobbie JE, Daley RJ, Jasper S . (1977). Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33: 1225–1228.
Hoffmann S, Otto C, Kurtz S, Sharma CM, Khaitovich P, Vogel J et al. (2009). Fast mapping of short sequences with mismatches, insertions and deletions using index structures. PLoS Comp Biol 5: e1000502.
Holert J, Hahnke S, Cypionka H . (2011). Influence of light and anoxia on chemiosmotic energy conservation in Dinoroseobacter shibae. Environ Microbiol Rep 3: 136–141.
Jarrell KF, McBride MJ . (2008). The surprisingly diverse ways that prokaryotes move. Nat Rev Microbiol 6: 466–476.
Jurgenson CT, Begley TP, Ealick SE . (2009). The structural and biochemical foundations of thiamin biosynthesis. Annu Rev Biochem 78: 569–603.
Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M et al. (2007). KEGG for linking genomes to life and the environment. Nucleic Acids Res 36: D480–D484.
Kimura H, Young CR, Martinez A, DeLong EF . (2011). Light-induced transcriptional responses associated with proteorhodopsin-enhanced growth in a marine flavobacterium. ISME J 5: 1641–1651.
Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N et al. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079.
Linke B, Giegerich R, Goesmann A . (2011). Conveyor: a workflow engine for bioinformatic analyses. Bioinformatics 27: 903–911.
Lois LM, Campos N, Putra SR, Danielsen K, Rohmer M, Boronat A . (1998). Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of D-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc Natl Acad Sci USA 95: 2105–2110.
Martinez A, Bradley AS, Waldbauer JR, Summons RE, DeLong EF . (2007). Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc Natl Acad Sci USA 104: 5590–5595.
Paerl RW, Bertrand EM, Allen AE, Palenik B, Azam F . (2015). Vitamin B1 ecophysiology of marine picoeukaryotic algae: Strain-specific differences and a new role for bacteria in vitamin cycling. Limnol Oceanogr 60: 215–228.
Palovaara J, Akram N, Baltar F, Bunse C, Forsberg J, Pedrós-Alió C et al. (2014). Stimulation of growth by proteorhodopsin phototrophy involves regulation of central metabolic pathways in marine planktonic bacteria. Proc Natl Acad Sci USA 111: E3650–E3658.
Riedel T, Gómez-Consarnau L, Tomasch J, Martin M, Jarek M, González JM et al. (2013). Genomics and Physiology of a marine flavobacterium encoding a proteorhodopsin and a xanthorhodopsin-like protein. PLoS ONE 8: e57487.
Riedel T, Tomasch J, Buchholz I, Jacobs J, Kollenberg M, Gerdts G et al. (2010). Constitutive expression of the proteorhodopsin gene by a flavobacterium strain representative of the proteorhodopsin-producing microbial community in the North Sea. Appl Environ Microbiol 76: 3187–3197.
Robinson M, Smyth G . (2007). Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 23: 2881–2887.
Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S et al. (2007). The Sorcerer II Global Ocean Sampling Expedition: northwest Atlantic through eastern tropical Pacific. PLoS Biol 5: e77.
Sabehi G, Loy A, Jung K-H, Partha R, Spudich JL, Isaacson T et al. (2005). New insights into metabolic properties of marine bacteria encoding proteorhodopsins. PLoS Biol 3: e273.
Sabehi G, Massana R, Bielawski JP, Rosenberg M, DeLong EF, Béjà O . (2003). Novel proteorhodopsin variants from the Mediterranean and Red seas. Environ Microbiol 5: 842–849.
Saier MH . (2000). A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev 64: 354–411.
Sañudo-Wilhelmy SA, Cutter LS, Durazo R, Smail EA, Gómez-Consarnau L, Webb EA et al. (2012). Multiple B-vitamin depletion in large areas of the coastal ocean. Proc Natl Acad Sci USA 109: 14041–14045.
Sañudo-Wilhelmy SA, Gómez-Consarnau L, Suffridge C, Webb EA . (2014). The role of B vitamins in marine biogeochemistry. Annu Rev Marine Sci 6: 339–367.
Shiba T, Simidu U, Taga N . (1979). Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl Environ Microbiol 38: 43–45.
Steindler L, Schwalbach MS, Smith DP, Chan F, Giovannoni SJ . (2011). Energy starved Candidatus Pelagibacter Ubique substitutes light-mediated ATP production for endogenous carbon respiration. PLoS ONE 6: e19725.
Stingl U, Desiderio RA, Cho JC, Vergin KL, Giovannoni SJ . (2007). The SAR92 clade: an abundant coastal clade of culturable marine bacteria possessing proteorhodopsin. Appl Environ Microbiol 73: 2290–2296.
Tang YZ, Koch F, Gobler CJ . (2010). Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc Natl Acad Sci USA 107: 20756–20761.
Tang K, Jiao N, Liu K, Zhang Y, Li S . (2012). Distribution and Functions of TonB-Dependent Transporters in Marine Bacteria and Environments: Implications for Dissolved Organic Matter Utilization. PLoS ONE 7: e41204.
Taylor BL, Zhulin IB . (1999). PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev 63: 479–506.
Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, Bennke CM et al. (2012). Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336: 608–611.
Thorvaldsdottir H, Robinson JT, Mesirov JP . (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinformatics 14: 178–192.
Tjaden B . (2015). De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol 16: 1.
Tomasch J, Gohl R, Bunk B, Diez MS, Wagner-Döbler I . (2011). Transcriptional response of the photoheterotrophic marine bacterium Dinoroseobacter shibae to changing light regimes. ISME J 5: 1957–1968.
Voet D, Voet JG . (2004) Biochemistry. Wiley & Sons: Hoboken, NJ, USA.
Wang Z, O'Shaughnessy TJ, Soto CM, Rahbar AM, Robertson KL, Lebedev N et al. (2012). Function and regulation of Vibrio campbellii proteorhodopsin: acquired phototrophy in a classical organoheterotroph. PLoS ONE 7: e38749.
Wong KR, Buckley JT . (1989). Proton motive force involved in protein transport across the outer-membrane of Aeromonas salmonicida. Science 246: 654–656.
Winkler WC, Breaker RR . (2005). Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59: 487–517.
Yoon JH, Kang SJ, Lee CH, Oh TK . (2005). Dokdonia donghaensis gen. nov., sp. nov., isolated from sea water. Int J Syst Evol Microbiol 55: 2323–2328.
Yoshizawa S, Kumagai Y, Kim H, Ogura Y, Hayashi T, Iwasaki W et al. (2014). Functional characterization of flavobacteria rhodopsins reveals a unique class of light-driven chloride pump in bacteria. Proc Natl Acad Sci USA 111: 6732–6737.
Acknowledgements
We thank Christopher Suffridge for the vitamin-B1 quantification in the growth medium. The manuscript was improved by the comments of five anonymous reviewers and the editor. This work was supported by the Marie Curie Actions–International Outgoing Fellowships (project 253970). Additional funding was provided by the Spanish Ministry of Science and Innovation (project CTM2013-48292-C3-3-R), the National Science Foundation (grants OCE1136818, OCE1335269 and OCE1435666), the Gordon and Betty Moore Foundation Marine Microbiology Initiative award 3779 and the German Research Organization (DFG), Collaborative Research Grant TRR51 Roseobacter.
Author contributions
LG-C and IW-D conceived and designed the experiments; LG-C and TR performed the experiments; LG-C and JMG analyzed the data; LG-C, JMG, IW-D, JAF and SJ contributed reagents/materials/analysis tools; LG-C, JMG, TR, SAS-W, IW-D and JAF contributed to the writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on The ISME Journal website
Rights and permissions
About this article
Cite this article
Gómez-Consarnau, L., González, J., Riedel, T. et al. Proteorhodopsin light-enhanced growth linked to vitamin-B1 acquisition in marine Flavobacteria. ISME J 10, 1102–1112 (2016). https://doi.org/10.1038/ismej.2015.196
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2015.196
This article is cited by
-
Effects of Light and Dark Conditions on the Transcriptome of Aging Cultures of Candidatus Puniceispirillum marinum IMCC1322
Journal of Microbiology (2024)
-
Rhodopsin-mediated nutrient uptake by cultivated photoheterotrophic Verrucomicrobiota
The ISME Journal (2023)
-
Dissecting Light Sensing and Metabolic Pathways on the Millimeter Scale in High-Altitude Modern Stromatolites
Microbial Ecology (2023)
-
Transcriptome architecture and regulation at environmental transitions in flavobacteria: the case of an important fish pathogen
ISME Communications (2021)
-
In situ light responses of the proteorhodopsin-bearing Antarctic sea-ice bacterium, Psychroflexus torques
The ISME Journal (2017)