Abstract
An anaerobic nitrate-reducing enrichment culture was established with a cyclic saturated petroleum hydrocarbon, cyclohexane, the fate of which in anoxic environments has been scarcely investigated. GC–MS showed cyclohexylsuccinate as a metabolite, in accordance with an anaerobic enzymatic activation of cyclohexane by carbon–carbon addition to fumarate. Furthermore, long-chain cyclohexyl-substituted cell fatty acids apparently derived from cyclohexane were detected. Nitrate reduction was not only associated with cyclohexane utilization but also with striking depletion of added ammonium ions. Significantly more ammonium was consumed than could be accounted for by assimilation. This indicated the occurrence of anaerobic ammonium oxidation (anammox) with nitrite from cyclohexane-dependent nitrate reduction. Indeed, nitrite depletion was stimulated upon further addition of ammonium. Analysis of 16S rRNA genes and subsequent cell hybridization with specific probes showed that approximately 75% of the bacterial cells affiliated with the Geobacteraceae and approximately 18% with Candidatus ‘Brocadia anammoxidans’ (member of the Planctomycetales), an anaerobic ammonium oxidizer. These results and additional quantitative growth experiments indicated that the member of the Geobacteraceae reduced nitrate with cyclohexane to nitrite and some ammonium; the latter two and ammonium added to the medium were scavenged by anammox bacteria to yield dinitrogen. A model was established to quantify the partition of each microorganism in the overall process. Such hydrocarbon oxidation by an alleged ‘denitrification’ (‘pseudo-denitrification’), which in reality is a dissimilatory loop through anammox, can in principle also occur in other microbial systems with nitrate-dependent hydrocarbon attenuation.
Similar content being viewed by others
Introduction
Cyclic saturated hydrocarbons (cycloalkanes) with or without alkyl substituents are common in petroleum (Tissot and Welte, 1984) and refined petroleum products. There are no explicit reports about cycloalkanes produced by living organisms, whereas unsaturated alkyl-substituted cyclic hydrocarbons such as monoterpenes are abundant and structurally diverse plant metabolites (Langenheim, 1994).
Biodegradation of cycloalkanes has been studied to a lesser extent than the biodegradation of n-alkanes and aromatic hydrocarbons (Prince, 2002; Van Hamme et al., 2003). Axenic bacterial strains hitherto reported to grow with cycloalkanes were aerobic. Most microbiological studies with cycloalkanes used cyclohexane as a model substrate (Stirling and Watkinson, 1977; Anderson et al., 1980; Trower et al., 1985; Rouvière and Chen, 2003). Cyclohexane is aerobically activated by cyclohexane monooxygenase yielding cyclohexanol, and further oxidized through cyclohexanone, caprolactone and adipate (Perry, 1984; Cheng et al., 2002).
Information about anaerobic biodegradation of cycloalkanes is scarce, whereas anaerobic degradation of n-alkanes has become well-established (for overview see reference Widdel et al., 2009). Complete degradation of ethylcyclopentane was shown with a sulfate-reducing enrichment culture (Rios-Hernandez et al., 2003). In addition, anaerobic biodegradation of alkylcyclopentanes, cyclohexane and alkylcyclohexanes under conditions of sulfate reduction or methanogenesis was observed in sediment samples amended with gasoline and gas condensate (Townsend et al., 2004). Anaerobic growth of pure cultures with cycloalkanes has not been observed (for example, Wilkes et al., 2003). An activation of alicyclic hydrocarbons by a radical-catalyzed addition to fumarate, analogous to n-alkane activation (Kropp et al., 2000; Rabus et al., 2001), was postulated on the basis of metabolite analysis in a sulfate-reducing enrichment culture with ethylcyclopentane (Rios-Hernandez et al., 2003). A denitrifying bacterium growing anaerobically with n-alkanes from petroleum co-metabolized cyclopentane and formed cyclopentylsuccinate (Wilkes et al., 2003); cyclopentane did not allow productive growth.
This study was undertaken to show bacteria potentially involved in cycloalkane degradation with nitrate as electron acceptor in anoxic surroundings. Cyclohexane (C6H12) was used as a prominent unsubstituted representative of cycloalkanes. Cyclohexane is a slightly water-soluble (saturation, 0.68 mM at 25 °C) and relatively volatile (bp, 80.7 °C) hydrocarbon (Eastcott et al., 1988; Dean, 1992). Containing exclusively apolar σ-bonds, cyclohexane is chemically rather unreactive. Still, from a thermodynamic point of view, cyclohexane is a slightly endergonic compound (ΔGf°=+26.7 kJ mol−1; for comparison: n-hexane, ΔGf°=−3.8 kJ mol−1; benzene, ΔGf°=+124.4 kJ mol−1). Cyclohexane is less toxic than many aromatic hydrocarbons, but slightly more toxic than volatile n-alkanes (Sikkema et al., 1994, 1995). Cyclohexane may enter the environment through petroleum and fuel spills, and due to its use as a solvent and raw chemical (Perry, 1984). It is expected that cyclohexane volatilizes easily in oxic environments at the air, whereas it may tend to persist in ‘closed’ anoxic environments. While straight-chain saturated and aromatic petroleum hydrocarbons under anoxic conditions in the presence of nitrate often promote enrichment of Betaproteobacteria (Rabus et al., 1999; Ehrenreich et al., 2000; Widdel et al., 2009), the presently obtained enrichment with cyclohexane and nitrate was dominated by a deltaproteobacterium. Growth of this bacterium, the likely degrader of cyclohexane, was accompanied by a member of the Planctomycetales, which was apparently responsible for scavenging nitrite by anaerobic ammonium oxidation (Musat, 2005). Here we present a detailed analysis of the enrichment culture and quantitative model of the microbial interaction.
Materials and methods
Source of organisms, media and cultivation techniques
Anoxic sediment from a lake (Bad Zwischenahner Meer, Germany) was used as a source of bacteria. A 5-ml volume of homogenized sediment was added per 50 ml of defined NaHCO3/CO2-buffered freshwater medium containing 5 mM NO3− and 4.7 mM NH4+ (Rabus and Widdel, 1995; Widdel, 2009) in a flat 100 ml bottle. The medium was overlaid with 3 ml of 2,2,4,4,6,8,8-heptamethylnonane (HMN; serving as inert carrier phase) containing 0.8% (v/v) cyclohexane. The bottles were sealed under an N2–CO2 mixture (9:1, v/v) with rubber stoppers and incubated at 28°C nearly horizontally so as to maximize the contact area between the medium and the hydrocarbon phase; orifices were kept below the medium surface to avoid contact between the hydrocarbon phase and the stopper (Rabus and Widdel, 1995). Sediment-containing cultures were briefly (few seconds) shaken once per day, whereas sediment-free subcultures were incubated with constant horizontal shaking (50–70 r.p.m.). The inoculum size for all subcultures was 10% (v/v). Growth experiments with highly enriched cultures were performed in flat 120-ml bottles with 100 ml culture medium overlaid with 5 ml HMN containing 0.2 or 0.5% (v/v) cyclohexane. In a growth experiment for determination of the electron balance, a limiting amount of 0.098 mmol (10.6 μl, at 20 °C; 0.21%, v/v) cyclohexane in the carrier phase (5 ml), 0.98 mmol nitrate and 0.95 mmol ammonium were added per 100 ml culture medium. Sterile bottles with cyclohexane and inoculated bottles lacking cyclohexane or nitrate were used as controls.
Determination of the cell mass ratio of two bacteria by microscopy
If in a mixed culture of three microbial types their microscopically counted numbers are N1, N2, and N3, the fraction by numbers of each type is N1/(N1+N2+N3), and so on (index in nominator from 1 to 3). Designating the average cell volume of each type V1cell, V2cell and V3cell, and assuming the same dry mass/volume conversion factor for each cell type, we obtain for the fraction by mass (dry mass share, biomass share) of each type:

Symbol m designates mass, mtot total biomass and index micr the microscopic approach (for distinction from other approaches). If bacterial type-1 and type-2 dominate by biovolume (minor biovolume contribution by the ‘accompanying’ type-3), we can write for the two dominant types

and

The volume of a spherical cell with diameter d is Vspherecell=πd3/6. The volume of a rod-shaped cell with rounded (hemispherical) ends, which has diameter d and total length l, is the sum of the volume of the cylindrical part (length, l−d) and the volume of a whole sphere, that is, Vrounded rodcell=πd2(3l−d)/12.
Chemical analyses
Nitrate and nitrite were measured by high-pressure liquid chromatography with UV (220 nm) detection as described by Rabus and Widdel (1995). Ammonium was determined colorimetrically by the indophenol formation reaction (Boltz and Taras, 1978).
Cyclohexane concentrations in the carrier phase (HMN) were measured by headspace analysis. The measurement is based on comparison of the cyclohexane content in gas phase samples from the culture and from calibrated samples in bottles, after gas phase equilibration with the carrier phase at constant temperature (28 °C). A 0.1-ml volume of gas phase was withdrawn with an N2-flushed gas-tight syringe and injected into a Shimadzu GC14B gas chromatograph (Shimadzu, Duisburg, Germany) equipped with a flame ionization detector and a 30 m Supel-Q PLOT fused silica capillary column (inner diameter 0.53 mm; Sigma Aldrich GmbH, Taufkirchen, Germany). Nitrogen was used as a carrier gas. The oven was operated isothermally at 140 °C, the detector at 280 °C and the injector at 150 °C.
Before metabolite extraction, the HMN phase was removed with a separatory funnel. Metabolites were extracted from an acidified culture volume of 400 ml as described previously (Wilkes et al., 2000; Rabus et al., 2001). Inoculated cultures without cyclohexane and sterile cultures containing cyclohexane were used as controls. Methylated culture extracts were analyzed by gas chromatography–mass spectrometry (GC–MS) using a type 5890 gas chromatograph (Hewlett Packard, Waldbronn, Germany) connected to a type 95SQ mass spectrometer (Finnigan MAT/ThermoFinnigan, Egelsbach, Germany) as described by Wilkes et al. (2000) and Rabus et al. (2001). Cyclohexylsuccinic acid as a standard was purchased from Sigma-Aldrich (Deisenhofen, Germany).
Clone library construction, sequencing and phylogenetic analysis
The nearly complete 16S rRNA gene was amplified by PCR using bacteria-specific primers (Musat et al., 2006). The 16S rRNA gene of the anammox bacteria was amplified with the primer pairs AMX368F—Univ1392R and Pla46F—Univ1392R (Schmid et al., 2005). PCR products were purified using the QIAquick Purification kit (Qiagen, Hilden, Germany) and cloned into the pCR4 vector (Invitrogen, Groningen, The Netherlands) according to the manufacturer's recommendation. The recombinant vectors were transformed into competent Escherichia coli Top10 cells (Invitrogen). The 16S rRNA gene libraries were screened by PCR using the primers M13F and M13R. Positive clones were sequenced using the ABI Prism BigDye Terminator v 3.0 cycle sequencing kit and an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The sequences were aligned to those in the SILVA rRNA database (Pruesse et al., 2007), and phylogenetic trees were constructed in ARB (Ludwig et al., 2004) by neighbor joining, maximum likelihood and maximum parsimony, and by applying different sets of filters. In addition, maximum likelihood phylogenetic trees were constructed using the RAxML algorithm (Stamatakis et al., 2008; http://phylobench.vital-it.ch/raxml-bb). The sequence data have been deposited in the DDBJ, EMBL and GenBank databases under accession numbers GU230600 and GU230601.
Fluorescence in situ hybridization
For fluorescence in situ hybridization (FISH), 1.0 ml from the enrichment culture was fixed with paraformaldehyde (final concentration, 20 g l−1) for 3 h at 4°C, washed twice with 1 × phosphate-buffered saline (10 mM sodium phosphate of pH 7.2, 130 mM NaCl) and stored in 1 × phosphate-buffered saline–ethanol (1:1) at −20 °C. For FISH, aliquots were filtered onto 0.2-μm pore GTTP polycarbonate filters (Millipore, Eschborn, Germany). Cells were hybridized with 16S rRNA-targeted probes, additionally stained with 4′,6′-diamidino-2-phenylindole (DAPI) and microscopically counted as described previously (Snaidr et al., 1997). FISH with catalyzed reporter deposition (CARD-FISH) using the probe AMX820 specific for the anammox bacteria (Schmid et al., 2001) was performed as described by Pernthaler et al. (2002). Values were corrected for the signals counted using the nonsense probe NON338. The oligonucleotide probes used in this study, GEO825 (30% formamide; Lowe et al., 2000), EUB338 (Amann et al., 1990), AMX368 (Schmid et al., 2000) and AMX820 (Schmid et al., 2001), were purchased from Biomers GmbH (Germany; www.biomers.net).
Results and discussion
Enrichment and analysis of products
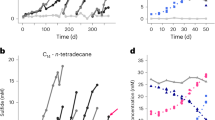
Enrichment of anaerobic cyclohexane-degrading bacteria was attempted with freshwater sediment (mud) incubated under heptamethylnonane (inert carrier phase) with 0.8% cyclohexane in the presence of nitrate as electron acceptor. Production of gas (detectable by a volumetric device; Widdel, 2009), which was presumably nitrogen, indicated bacterial activity. When gas production ceased, new nitrate was added. Gas production in these early states of enrichment always resumed upon nitrate addition. During approximately 8 weeks, gas production in cyclohexane-free controls was essentially the same as in incubations with cyclohexane, indicating the presence of nitrate-scavenging electron donors in the organic-rich inoculum. After 8–9 weeks, however, cultures with cyclohexane produced more gas than the controls. Repeated transfers to new media resulted in a mud-free, strictly cyclohexane-dependent enrichment culture consisting mainly of rod-shaped cells. These cultures consumed 5 mM nitrate within approximately 3 weeks. Growth was always accompanied by gas production. The bacterial biomass that settled in the grown cultures was pink to reddish. The visible spectra of whole cells showed an absorption maximum at 408 nm, which was indicative of cytochromes.
GC–MS of extracts from the cyclohexane-grown culture showed a mixture of organic acids that were not detectable in controls lacking cyclohexane. A metabolite with prominent fragment ions at m/z 114, 146, 155 and 197 was identified as cyclohexylsuccinic acid dimethyl ester on the basis of comparison with an authentic standard (Figure 1). This suggests that cyclohexane is anaerobically activated by addition to fumarate, similar as n--alkanes (Kropp et al., 2000; Rabus et al., 2001) and ethylcyclopentane (Rios-Hernandez et al., 2003). Furthermore, two other metabolites were tentatively identified (on the basis of the mass spectra) as 9-cyclohexylnonanoic acid and 11-cyclohexylundecanoic acid (Figure 2). Their formation can be explained by a pathway of cyclohexylsuccinate analogous to the suggested pathway of n-alkanes involving CoA-ligation, C-skeleton rearrangement and decarboxylation to 3-cyclohexylpropionyl-CoA (Wilkes et al., 2002, 2003); the latter undergoes chain elongation with C2-units during lipid synthesis (Figure 3). Such ω-cyclohexyl fatty acids have been also detected in oil samples and oil formation waters with high content of partly biodegraded oil (Rodrigues et al., 2005). Hence, ω-cyclohexyl fatty acids may indicate anaerobic degradation of cycloalkanes under in situ conditions.
The proposed pathway for the anaerobic degradation of cyclohexane. The circled letters indicate compounds detected as methyl esters. Cyclohexane (a) is activated by addition to fumarate (b), yielding cyclohexylsuccinate (c), which may then be activated to cyclohexylsuccinyl-CoA (d). The latter may undergo rearrangement of the C-skeleton to (cyclohexylmethyl)malonyl-CoA (e), which through decarboxylation (or transcarboxylation) yields cyclohexylpropionyl-CoA (f). The bulk of the latter is degraded by regular β-oxidation through 3-oxo-3-cyclohexylpropionyl-CoA (g), cyclohexylcarboxyl-CoA (h), and ring cleavage to yield acetyl-CoA as well as new fumarate for the activation reaction. Some cyclohexylpropionyl-CoA (f) may contribute to the synthesis of cellular fatty acids by addition of C2-units yielding 9-cyclohexylnonanoate (i) and 11-cyclohexylundecanoate (j).
Even though nitrate was added in sub-stoichiometric amounts relative to cyclohexane, the highly enriched mud-free cultures, viz. the later stages of enrichment, often did not resume growth and gas production upon new nitrate addition. Products of nitrate reduction were therefore analyzed, in particular to detect possible accumulation of nitrite as a potentially growth-inhibiting compound. Nitrite accumulation was indeed observed after addition of new nitrate to stationary cultures. Nitrous oxide (N2O) and ammonium as possible products of nitrate reduction were not detected. By contrast, the initially added ammonium (4.7 mmol per liter) was consumed to depletion during the reduction of nitrate (not shown). Such depletion could not be explained by ammonium assimilation into cell mass. The optical density (OD660) at the time of ammonium depletion was approximately 0.12, corresponding to no more than approximately 40 mg cell dry mass per liter (estimated from correlations between biomass yields and OD660 given by Rabus and Widdel, 1996). According to an approximate bulk formula for cell mass (C4H8O2N; van Dijken and Harder, 1975) with 14% N (by mass), the estimated cell synthesis in this enrichment needed only 0.4 mmol NH4+ per liter. This is less than 10% of the observed ammonium consumption. Such strong ammonium depletion can be only explained by a dissimilatory process, the known anaerobic ammonium oxidation (anammox) with nitrite. This process is catalyzed by specialized members of the Planctomycetales (Mulder et al., 1995; Jetten et al., 2009). Attempts to purify cyclohexane-degrading nitrate reducers and anammox bacteria to further verify the assumed reactions in the enrichment culture were not successful. To gain more insights into the bacterial types and reactions, we therefore analyzed the dominant phylotypes on the basis of 16S rRNA sequences and performed physiological experiments with the enrichment culture.
Phylotypes in the enrichment culture
The 16S rRNA gene library generated using standard bacterial primers was dominated by a sequence characteristic of the family Geobacteraceae of the Deltaproteobacteria (clone type I; Figure 4a). The closest cultivated relatives were Geobacter sp. FRC-32 and Geobacter uraniireducens (sequence similarity, 96.0 and 95.5%, respectively). The apparent abundance of this phylotype in the enrichment culture was corroborated by FISH using a 16S rRNA-targeted fluorescent probe specific for the Geobacteraceae. The Geobacter-related phylotype accounted for approximately 75% of the (total) cell number determined by DAPI staining (Figures 5a and b). Because of the above indication of anaerobic nitrite-dependent ammonium oxidation, two primer pairs for 16S rRNA genes of anammox bacteria were also applied. A phylotype affiliating with the anammox cluster of the Planctomycetales (clone type II; Figure 4b) was indeed retrieved; it was most closely related to Candidatus ‘Brocadia anammoxidans’ (93.6% sequence similarity). Hybridization with specific probes showed that cells belonging to the anammox phylotype accounted for up to 18% of the total cell number in the enrichment culture (Figures 5c and d).
Phylogenetic affiliation of the dominant microorganisms in the enrichment culture with (a) clone type I among the Geobacteraceae and (b) clone type II among anammox bacteria (Planctomycetales). Only nearly full-length sequences (>1300 bp) were used for the calculation. The sequences retrieved in this study are indicated in boldface. The numbers adjacent to nodes indicate maximum likelihood bootstrap values higher than 50%. The scale bars represent 10% estimated sequence divergence.
FISH of the cyclohexane- and nitrate-utilizing enrichment culture with the GEO825 probe (a) showed dominance of Geobacteraceae related organisms, accounting for up to 75% of the DAPI-stained cells (b). Hybridization with the AMX368 probe (c) indicated that anammox-related organisms represented up to 18% of the DAPI-stained cells (d). Scale bar: 10 μm (applicable to all images). DAPI, 4′,6′-diamidino-2-phenylindole; FISH, fluorescence in situ hybridization.
Assignment of anaerobic ammonium oxidation in our enrichment to the detected Planctomycetales would leave the assignment of cyclohexane degradation to the Geobacter-related phylotype. Anammox bacteria with their special nitrogen catabolism are not known to use hydrocarbons. However, there is much biodegradative potential in the genus Geobacter and other Deltaproteobacteria towards low-molecular-mass organic compounds. Iron(III)-reducing Geobacter species have been shown to anaerobically oxidize aromatic compounds (Lovley et al., 1989; Wischgoll et al., 2005). Several sulfate-reducing Deltaproteobacteria oxidize n-alkanes or aromatic hydrocarbons (for overview see reference Widdel et al., 2009). Geobacter species and some sulfate-reducing bacteria may also reduce nitrate, even though these anaerobes are usually not directly enriched with nitrate. Enrichments with hydrocarbons (n-alkanes, alkylbenzenes) and nitrate very often selected true denitrifiers (viz. that reduce NO3− to N2) of the Betaproteobacteria (for example, Rabus et al., 1999; Ehrenreich et al., 2000). Physiological and genomic studies (Lovley and Phillips, 1988; http://img.jgi.doe.gov) suggest that reduction of nitrate by Geobacteraceae, if they reduce it beyond the level of nitrite, produces ammonium, as in sulfate-reducing Deltaproteobacteria (Widdel and Pfennig, 1992; Simon, 2002). The anammox bacteria may therefore be fostered by the ammonium added to the medium as well as by the ammonium and nitrite produced during cyclohexane oxidation.
Time course of substrate consumption and product formation
The fate of cyclohexane, nitrate and ammonium, as well as formation of nitrite and optical cell density, was monitored in a time-course experiment (Figures 6a and b). As long as ammonium was present, nitrite appeared only transiently and at low concentration. If new nitrate was added, nitrite accumulated to higher concentration as soon as the residual ammonium was depleted (Figure 6b). But even if ammonium was no longer detectable, more nitrate was reduced than nitrite was formed, indicating that a part of formed nitrite was further reduced by the cyclohexane-degrading bacteria, or by accompanying bacteria that may be nourished by metabolic products excreted by the cyclohexane degrader. New addition of ammonium resulted in rapid nitrite consumption (Figure 6b). Consumption of cyclohexane or ammonium was not observed in control incubations without nitrate. Also, ammonium was not consumed in inoculated cyclohexane-free controls containing nitrate (not shown).
Anaerobic consumption of cyclohexane, nitrate and ammonium, as well as formation and consumption of nitrite (as an intermediate), in the anaerobic enrichment culture. Experiments were performed in anoxic bottles with a culture volume of 100 ml and 5 ml heptamethylnonane as inert carrier for cyclohexane (the given values are relative to the aqueous phase). (a) Cell density (OD, ▪) increased only if cyclohexane (•) was present. In a cyclohexane-free control the cell density (□) remained constant. The main increase in OD occurred during the initial incubation period. Cyclohexane in a sterile control (○; tested at higher concentration) did not show significant disappearance. (b) In the same experiment, nitrate (♦) consumption was accompanied by ammonium (▴) depletion. Addition of new nitrate after 15 days resulted in its partial reduction and some accumulation of nitrite (×). No growth was observed during this period. New ammonium addition after 27 days caused consumption of nitrite and some growth.
Electron balance and proportion of reactions and cell types in the overall process
A net electron balance was determined using a culture provided with a small, defined amount of cyclohexane. The results are summarized in Table 1. In this culture, the added cyclohexane was completely consumed. Absence of N2O and presence of anammox bacteria leave N2 as the only end product of nitrate reduction and ammonium oxidation. Calculation showed that electrons from consumed cyclohexane and ammonium essentially matched the electrons channeled into cell synthesis and into reduction of nitrate (through nitrite).
As suggested by the time-course experiment (Figure 6), nitrite formed by the nitrate reducer (the Geobacter type) is not only scavenged by anammox bacteria but also further reduced by the nitrate reducer itself to yield ammonium. The proportions of nitrite entering these two branches are in principle variable and influence the extent of coupled anammox. To calculate the proportions of the reactions in the overall process, we formulate the two reactions of the nitrate reducer, both starting with nitrate (Equations 4, 5), as well as the anammox reaction (Equation 6):



Strictly speaking, energy changes in Equations 4–6 represent changes of chemical potential. We assume that the nitrogen of NO3− and NH4+ consumed in the culture always ends up as N2, irrespective of whether the intermediate NO2− enters anammox directly or indirectly after further reduction to NH4+. If the measured amounts (in mol) of NO3− and NH4+ consumed from the medium are termed a and b, respectively, they must yield [(a+b)/2] N2. Because equimolar amounts of nitrite and ammonia must react in anammox (Equation 6), an amount of [(a+b)/2] NO2− derived from [(a+b)/2] NO3− must finally react with [(a+b)/2] NH4+. Ammonium for anammox has two sources: NH4Cl added to the medium and NH4+ formed by the nitrate reducer. If from the ammonium initially present in the medium the (measured) amount b NH4+ has been consumed, the proportion [(a+b)/2]−b=[(a−b)/2] NH4+ must have been provided by the cyclohexane degrader through complete reduction of [(a−b)/2] NO3− (Figure 7). With such generally expressed amounts (in mol), the two branches of nitrate reduction and the associated free energy changes (indices: nire1, nire2) are represented by Equations (7) and (8).


A functional model of the cyclohexane-degrading nitrate-reducing enrichment culture. The factors a and b denote amounts in mol. The Geobacter type oxidizes cyclohexane and reduces nitrate mainly to nitrite, and to a lesser extent to ammonium. Anammox bacteria use the produced nitrite for oxidation of ammonium from further nitrate reduction and from the surroundings to N2. Nitrite may inhibit the Geobacter type. For convenience, the scheme does not depict the stoichiometry of cyclohexane oxidation to CO2 (see text, Equation (9)). Red, reduction.
Total nitrate reduction (index: nire) is their sum given by Equation (9).

The anammox reaction (index: anam) is represented by Equation (10).

Addition of Equations (9) and (10) yield Equation (11) for the total reaction in the culture (formed and consumed nitrite cancel each other; the amounts of ammonium are combined on the reactant side).

In the incubation experiment using a culture volume of 100 ml, a consumption of a=0.94 mmol NO3− and b=0.67 mmol NH4+ was measured (Table 1). Of the 0.94 mmol NO2− formed as an intermediate, (0.94+0.67)/2=0.805 mmol NO2− were thus directly used by anammox to oxidize 0.805 mmol NH4+, whereas 0.135 mmol NO2− were further reduced to 0.135 mmol NH4+. Hence, the bulk (86%) of the NO2− produced was scavenged by anammox bacteria.
The free energies available from nitrate reduction and anammox in the incubation experiment were ΔG°nire=−178 kJ and ΔG°′anam=−288 kJ, respectively. The free energy change of the total process was ΔG°′tot=−466 kJ. Hence, the free energy share between nitrate reduction and anammox is as follows:


The biomass share of the two bacteria in the culture is not necessarily according to their free energy share. The bacteria may differ with respect to their efficiencies of energy conservation and use of conserved energy for biosynthesis. The biomass share can be estimated from the presently consumed amounts of the reactants (substrates) and from growth yields (cell dry mass per mol substrate) reported in the literature. In addition, the biomass share is derived independently from cell numbers and their biovolumes determined microscopically. For comparison, we use both approaches.
Growth and growth yields of a nitrate reducer with cyclohexane have not been reported. As a rough estimate, we used a reported growth yield of a Geobacter species relative to the electrons channeled from benzoate oxidation into Fe(III) reduction, the value being 1.56 g dry mass per mol electrons (Champine et al., 2000); for convenience, we further assume that the yield relative to substrate-derived electrons is the same for nitrate reduction to nitrite and nitrate reduction to ammonium. The examined culture dissimilated a total of 4.6 mmol electrons of which 2.0 mmol electrons were from added ammonium (Table 1). The amount of electrons derived from cyclohexane for dissimilation was thus 4.6−2.0=2.6 mmol. Essentially the same value is obtained from the term [(5a−3b)/36] C6H12 in Equation (9), using the above values for a and b, and 36 mol electrons derived per mol C6H12. The resulting dry mass of the nitrate reducer in the culture (100 ml) was thus mnire=4 mg. The growth yield of anammox bacteria is 1.59 g dry mass per mol ammonium (calculated from Kuenen, 2008). Anammox cells in the culture consumed a calculated amount of 0.805 mmol NH4+, which would allow a biomass synthesis of manam=1.3 mg. The resulting total biomass in the culture was mtot=5.3 mg. The predicted (index: pred) cell dry mass share between the nitrate-reducing and the anammox bacterium is thus as follows:


To calculate the biomass share from the fractions of microscopically determined cell numbers, we estimated the average cell volume of the nitrate reducer (Vnirecell=0.29 μm3) and the anammox bacterium (Vanamcell=0.41 μm3) by examining 20 cells of each type. These were the cell volumes after fixation and staining, which causes shrinking of the cells. However, if we assume that the cell volumes shrink by the same factor, Equation (2) can be applied. This yields the following biomass share:


These values are in good agreement with the above ones predicted from substrate consumption.
Results show that the Geobacter type dominates by mass, even though it shares the smaller proportion of the total free energy. The reason lies in a more efficient use of the free energy for cell synthesis in comparison with the energy use in the special lithoautotrophic metabolism of anammox bacteria.
Conclusions
The catabolic processes suggested to occur in our enrichment culture (Figure 7) possibly represent a delicate interaction. On the one hand, the nitrate-reducing Geobacter type and anammox bacteria may compete for nitrite. On the other hand, nitrite accumulation may inhibit the nitrate reducer (as the time course experiment suggests; Figure 6). It is therefore possible that the nitrate reducer benefits from or depends on nitrite removal. This would offer an explanation for the relatively slow growth of the present enrichment culture in comparison with that of other hydrocarbon-utilizing denitrifiers; with similar inoculum sizes, the latter grew within 8 days or less (Rabus and Widdel, 1995; Ehrenreich et al., 2000). The cyclohexane-degrading nitrate reducer may thus develop only if nitrite-scavenging anammox bacteria, which generally grow slowly (Kuenen, 2008), are co-enriched. In other words, their co-enrichment would be compulsory. A different explanation would be that the cyclohexane-degrading nitrate reducer is also a slowly growing organism, like anammox bacteria. In this case, enrichment of the latter would be coincidental and due to the long incubation time needed to grow the cyclohexane degrader. Only isolation and a physiological study of the Geobacter type would clarify its properties and the mode of interaction in the enrichment culture.
One may expect that also in situ degradation of petroleum hydrocarbons with nitrate in contaminated anoxic soil or sediment is associated with anammox under certain conditions. A prerequisite for such association would be that anammox bacteria are not inhibited by petroleum, viz. that they tolerate various hydrophobic organic compounds acting on biomembranes. This is likely because anammox bacteria were shown to tolerate phenolic waste water (Toh and Ashbolt, 2002).
This study shows that anaerobic biodegradation of organic compounds coupled to NO3− reduction may, at first glance, pretend denitrification, but in reality yield N2 through a ‘loop’ through anammox (‘pseudo-denitrification’). Such dissimilatory conversion of NO3− to N2 without involvement of true denitrification has been shown by isotope labeling studies using highly enriched cells of anammox bacteria alone (Kartal et al., 2007). If formate was provided as electron donor, cells catalyzed the reduction of NO3− to NO2− and NH4+, which were further converted to N2.
Finally, a generalized net process may be formulated for the anaerobic oxidation of an organic compound, CcHhOo (for convenience without N and electrical charge), coupled to non-denitrifying nitrate reduction in combination with anammox:

A special situation would be a reaction without dissimilatory consumption of external ammonium (b=0; see also Equation (11) and Figure 7), which as a bulk reaction is indistinguishable from true denitrification. In this case, the nitrate reducer forms equimolar amounts of nitrite and ammonium. This is analogous to the reaction shown in incubations with anammox cells (Kartal et al. 2007). If there is net production rather than net consumption of ammonium (formally −a<b<0 in Equation (18), or NH4+ appearing on the product side with a>b>0), the nitrate reducer produces more NH4+ than NO2−, and anammox is involved to a lesser extent. If NO3− is completely reduced to NH4+ (b=−a, or NH4+ on the product side with b=a), there is no longer a possibility for anammox (and N2 production) to occur.
Accession codes
References
Amann RI, Krumholz L, Stahl DA . (1990). Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol 172: 762–770.
Anderson MS, Hall RA, Griffin M . (1980). Microbial metabolism of alicyclic hydrocarbons: cyclohexane catabolism by a pure strain of Pseudomonas sp. J Gen Microbiol 120: 89–94.
Boltz DF, Taras MJ . (1978). Nitrogen. In: Boltz DF, Howell JA (eds). Colorimetric Determination of Nonmetals. Wiley: New York, NY, USA. pp 197–251.
Champine JE, Underhill B, Johnston JM, Lilly WW, Goodwin S . (2000). Electron transfer in the dissimilatory iron-reducing bacterium Geobacter metallireducens. Anaerobe 6: 187–196.
Cheng Q, Thomas SM, Rouvière PE . (2002). Biological conversion of cyclic alkanes and cyclic alcohols into dicarboxylic acids: biochemical and molecular basis. Appl Microbiol Biotechnol 58: 704–711.
Dean JA . (1992). Lange's Handbook of Chemistry. McGraw-Hill: New York, NY, USA.
Eastcott L, Shiu WY, Mackay D . (1988). Environmentally relevant physical–chemical properties of hydrocarbons: a review of data and developments of simple correlations. Oil Chem Pollut 4: 191–216.
Ehrenreich P, Behrends A, Harder J, Widdel F . (2000). Anaerobic oxidation of alkanes by newly isolated denitrifying bacteria. Arch Microbiol 173: 58–64.
Jetten MSM, Niftrik LV, Strous M, Kartal B, Keltjens JT, Op den Camp HJM . (2009). Biochemistry and molecular biology of anammox bacteria. Crit Rev Biochem Mol Biol 44: 65–84.
Kartal B, Kuypers MMM, Lavik G, Schalk J, Op den Camp HJM, Jetten MSM et al. (2007). Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ Microbiol 9: 635–642.
Kropp KG, Davidova IA, Suflita JM . (2000). Anaerobic oxidation of n-dodecane by an addition reaction in a sulfate-reducing bacterial enrichment culture. Appl Environ Microbiol 66: 5393–5398.
Kuenen JG . (2008). Anammox bacteria: from discovery to application. Nat Rev Microbiol 6: 320–326.
Langenheim JH . (1994). Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20: 1223–1280.
Lovley DR, Baedecker MJ, Lonergan DJ, Cozzarelli IM, Phillips EJP, Siegel DI . (1989). Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339: 297–300.
Lovley DR, Phillips EJP . (1988). Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol 54: 1472–1480.
Lowe KL, Dichristina TJ, Roychoudhury AN, Van Cappellen P . (2000). Microbiological and geochemical characterization of microbial Fe(III) reduction in salt marsh sediments. Geomicrobiol J 17: 163–178.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
Mulder A, van de Graaf AA, Robertson LA, Kuenen JG . (1995). Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol Ecol 16: 177–183.
Musat F . (2005). Physiological investigations of aerobic petroleum degradation in marine sediment microcosms. PhD thesis, University of Bremen, Germany.
Musat N, Werner U, Knittel K, Kolb S, Dodenhof T, Van Beusekom JEE et al. (2006). Microbial community structure of sandy intertidal sediments in the North Sea, Sylt-Rømø Basin, Wadden Sea. Syst Appl Microbiol 29: 333–348.
Pernthaler A, Pernthaler J, Amann R . (2002). Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Environ Microbiol 68: 3094–3101.
Perry JJ . (1984). Microbial metabolism of cyclic alkanes. In: Atlas RM (ed). Petroleum Microbiology. Macmillan: New York, NY, USA. pp 61–99.
Prince RC . (2002). Petroleum and other hydrocarbons, biodegradation of. In: Bitton G (ed). Encyclopedia of Environmental Microbiology. John Wiley: New York, NY, USA. pp 2402–2416.
Pruesse E, Quast C, Knittel K, Fuchs B, Ludwig W, Peplies J et al. (2007). SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35: 7188–7196.
Rabus R, Widdel F . (1995). Anaerobic degradation of ethylbenzene and other aromatic hydrocarbons by new denitrifying bacteria. Arch Microbiol 163: 96–103.
Rabus R, Widdel F . (1996). Utilization of alkylbenzenes during anaerobic growth of pure cultures of denitrifying bacteria on crude oil. Appl Environ Microbiol 62: 1238–1241.
Rabus R, Wilkes H, Behrends A, Armstroff A, Fischer T, Pierik AJ et al. (2001). Anaerobic initial reaction of n-alkanes in a denitrifying bacterium: evidence for (1-methylpentyl)succinate as initial product and for involvement of an organic radical in n-hexane metabolism. J Bacteriol 183: 1707–1715.
Rabus R, Wilkes H, Schramm A, Harms G, Behrends A, Amann R et al. (1999). Anaerobic utilization of alkylbenzenes and n-alkanes from crude oil in an enrichment culture of denitrifying bacteria affiliating with the β-subclass of the Proteobacteria. Environ Microbiol 1: 145–157.
Rios-Hernandez LA, Gieg LM, Suflita JM . (2003). Biodegradation of an alycyclic hydrocarbon by a sulfate-reducing enrichment from a gas condensate-contaminated aquifer. Appl Environ Microbiol 69: 434–443.
Rodrigues D, de Vasconcellos S, Alves P, Nascimento L, de Abreu B, de Oliveira V et al. (2005). Relationship between cyclohexyl-alkanoic acids and the acidothermophilic bacterium Alicyclobacillus spp.:evidence from Brazilian oils. Org Geochem 36: 1443–1453.
Rouvière PE, Chen MW . (2003). Isolation of Brachymonas petroleovorans CHX, a novel cyclohexane-degrading β-proteobacterium. FEMS Microbiol Lett 227: 101–106.
Schmid M, Schmitz-Esser S, Jetten M, Wagner M . (2001). 16S-23S rDNA intergenic spacer and 23S rDNA of anaerobic ammonium-oxidizing bacteria: implications for phylogeny and in situ detection. Environ Microbiol 3: 450–459.
Schmid M, Twachtmann U, Klein M, Strous M, Juretschko S, Jetten M et al. (2000). Molecular evidence for genus level diversity of bacteria capable of catalyzing anaerobic ammonium oxidation. Syst Appl Microbiol 23: 93–106.
Schmid MC, Maas B, Dapena A, van de Pas-Schoonen K, van de Vossenberg J, Kartal B et al. (2005). Biomarkers for in situ detection of anaerobic ammonium-oxidizing (Anammox) bacteria. Appl Environ Microbiol 71: 1677–1684.
Sikkema J, de Bont JAM, Poolman B . (1994). Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269: 8022–8028.
Sikkema J, de Bont JAM, Poolman B . (1995). Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59: 201–222.
Simon J . (2002). Enzymology and bioenergetics of respiratory nitrite ammonification. FEMS Microbiol Rev 26: 285–309.
Snaidr J, Amann R, Huber I, Ludwig W, Schleifer K-H . (1997). Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl Environ Microbiol 63: 2884–2896.
Stamatakis A, Hoover P, Rougemont J . (2008). A rapid bootstrap algorithm for the RAxML web-servers. Syst Biol 75: 758–771.
Stirling LA, Watkinson RJ . (1977). Microbial metabolism of alicyclic hydrocarbons: isolation and properties of a cyclohexane-degrading bacterium. J Gen Microbiol 99: 119–125.
Tissot BP, Welte DH . (1984). Petroleum Formation and Occurence. Springer: Berlin, Germany.
Toh SK, Ashbolt NJ . (2002). Adaptation of anaerobic ammonium-oxidising consortium to synthetic coke-ovens wastewater. Appl Microbiol Biotechnol 59: 344–352.
Townsend GT, Prince RC, Suflita JM . (2004). Anaerobic biodegradation of alicyclic constituents of gasoline and natural gas condensate by bacteria from an anoxic aquifer. FEMS Microbiol Ecol 49: 129–135.
Trower MK, Buckland RM, Higgins R, Griffin M . (1985). Isolation and characterization of cyclohexane-metabolizing Xanthobacter sp. Appl Environ Microbiol 49: 1282–1289.
van Dijken JP, Harder W . (1975). Growth yields of microorganisms on methanol and methane. A theoretical study. Biotechnol Bioeng 17: 15–30.
Van Hamme JD, Singh A, Ward OP . (2003). Recent advances in petroleum microbiology. Microbiol Mol Biol Rev 67: 503–549.
Widdel F . (2009). Cultivation of anaerobic microorganisms with hydrocarbons as growth substrates. In: Timmis KN (ed). Handbook of Hydrocarbon and Lipid Microbiology. Springer: Heidelberg, Germany. pp 3787–3798.
Widdel F, Knittel K, Galushko A . (2009). Anaerobic hydrocarbon-degrading microorganisms: an overview. In: Timmis KN (ed). Handbook of Hydrocarbon and Lipid Microbiology. Springer: Heidelberg, Germany. pp 1997–2022.
Widdel F, Pfennig N . (1992). The genus Desulfuromonas and other Gram-negative sulfur-reducing eubacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds). The Prokaryotes. Springer: New York, NY, USA. pp 3379–3389.
Wilkes H, Boreham C, Harms G, Zengler K, Rabus R . (2000). Anaerobic degradation and carbon isotopic fractionation of alkylbenzenes in crude oil by sulphate-reducing bacteria. Org Geochem 31: 101–115.
Wilkes H, Kuhner S, Bolm C, Fischer T, Classen A, Widdel F et al. (2003). Formation of n-alkane- and cycloalkane-derived organic acids during anaerobic growth of a denitrifying bacterium with crude oil. Org Geochem 34: 1313–1323.
Wilkes H, Rabus R, Fischer T, Armstroff A, Behrends A, Widdel F . (2002). Anaerobic degradation of n-hexane in a denitrifying bacterium: further degradation on the initial intermediate (1-methylpentyl)succinate via C-skeleton rearrangement. Arch Microbiol 177: 235–243.
Wischgoll S, Heintz D, Peters F, Erxleben A, Sarnighausen E, Reski R et al. (2005). Gene clusters involved in anaerobic benzoate degradation of Geobacter metallireducens. Mol Microbiol 58: 1238–1252.
Acknowledgements
We thank Niculina Musat for help with whole-cell hybridization, Ramona Appel for technical assistance and an anonymous reviewer for stimulating comments. This work was supported by the Max-Planck-Gesellschaft and the European Community project MATBIOPOL (EVK3-CT1999-00010, 2000-2003; grant to FW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Musat, F., Wilkes, H., Behrends, A. et al. Microbial nitrate-dependent cyclohexane degradation coupled with anaerobic ammonium oxidation. ISME J 4, 1290–1301 (2010). https://doi.org/10.1038/ismej.2010.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.50
Keywords
This article is cited by
-
Fungal biotransformation of short-chain n-alkylcycloalkanes
Applied Microbiology and Biotechnology (2019)
-
Anaerobic Benzene Mineralization by Nitrate-Reducing and Sulfate-Reducing Microbial Consortia Enriched From the Same Site: Comparison of Community Composition and Degradation Characteristics
Microbial Ecology (2018)
-
Denitrification synergized with ANAMMOX for the anaerobic degradation of benzene: performance and microbial community structure
Applied Microbiology and Biotechnology (2017)
-
A Review on the Genetics of Aliphatic and Aromatic Hydrocarbon Degradation
Applied Biochemistry and Biotechnology (2016)