Abstract
Escherichia coli carrying a natural conjugative F-plasmid generates F-pili mating pairs, which is important for early biofilm formation. In this study, we investigated the effect of male-specific filamentous single stranded DNA bacteriophage (f1) and RNA bacteriophage (MS2) on the formation of biofilms by E. coli carrying a natural conjugative F-plasmid. We showed that the early biofilm formation was completely inhibited by addition of the f1 phage, but not the MS2 phage. This suggests that the tip of F-pili is the specific attachment site for mating pairs formation and the side of F-pili has a non-obligatory role during biofilm formation. The inhibitory effect of the f1 phage was dependent on the time of addition during the biofilm formation. No inhibitory effect was observed when the f1 phages were added to the mature biofilms. This resistant mechanism of the mature biofilms could be attributed to the biofilm-specific phenotypes representing that the F-pili mating pairs were already formed and then the curli production commenced during the biofilm maturation. The pre-formed mating pairs seemed to resist the f1 phages. Altogether, our results indicate a close relationship between the presence of conjugative plasmid and male-specific bacteriophages within sessile biofilm communities, as well as the possibility of using the male-specific bacteriophages to control biofilm formation.
Similar content being viewed by others
Main
It has been demonstrated that conjugative plasmids promote bacterial biofilm formation (Ghigo, 2001; Reisner et al., 2006a, 2006b). Escherichia coli strains carrying a conjugative F-plasmid are the best characterized strains among those forming biofilms. Many other conjugative plasmids also contribute directly to biofilm formation upon derepression of the conjugative-factor leading to constitutive F-pili expression (Ghigo, 2001). In case of a natural conjugative F-plasmid carrying E. coli, F-pili mediate cell–cell interactions (Pereira et al., 2010) and consequently induce the capsular exopolysaccharide, colonic acid and the proteinacous extracellular amyloidal fiber (that is, curli), which stabilized a mature biofilm structure (May and Okabe, 2008). Cell–cell mating pair commences when the tips of F-pili make contact with another cells at close proximity (Frost et al., 1994). Besides mating pair formation by F-pili, generally, male-specific filamentous single stranded DNA bacteriophages, such as f1, fd or M13, bind specifically the tips of F-pili, whereas RNA bacteriophages, such as MS2 or Qβ, bind only along the side of F-pili (Ou, 1973). Notably, the RNA bacteriophages cause bacterial cell lysis after infection, but the filamentous single stranded DNA bacteriophages are non-lytic virions. Despite a number of studies on bacterial biofilms and bacteriophages present in environments, only a few studies have addressed the effects of bacteriophage infection on E. coli biofilm formation. Exposure of E. coli carrying conjugative plasmid biofilms to male-specific bacteriophages also has not been investigated yet. Of these, the previous studies mainly focused on lytic-bacteriophages, such as λ, T4 or T7, which failed to penetrate through biofilms because of the presence of exopolymers (Doolittle et al., 1995, 1996; Corbin et al., 2001) and/or fimbriae (Lacqua et al., 2006). In addition, many lytic bacteriophages are unable to replicate efficiently in the F-plasmid carrying E. coli (Garcia and Molineux, 1995).
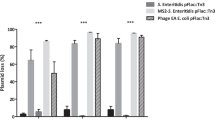
In order to investigate interaction between bacteria and male-specific bacteriophages within a biofilm matrix, we examined the effects of infection by bacteriophages, f1 and MS2 (either alone or in combination), on biofilm formation by E. coli carrying a natural repressed conjugative F-plasmid. Bacteriophages were purchased from the Department of Biotechnology, National Institute of Technology and Evaluation (Japan) and propagated as previously described (Ou, 1973). A natural F-plasmid (May and Okabe, 2008) was transformed into E. coli MG1655 (Blattner et al., 1997), which was used throughout in this study. In standard infection experiments with liquid cultures in polystyrene 96-well plates (TPP, Trasadingen, Switzerland), such as those shown in Figure 1a, bacteria from overnight cultures (usually grown on Luria-Bertani medium at 37 °C) were diluted with fresh medium at a final concentration of approximately 106 colony forming units per ml. To observe the effect of bacteriophage infection on the biofilm formation during the early bacterial attachment stage, the bacteriophage(s) was added at 0, 105, 106, 107 and 108 plaque forming unit (PFU) per ml, corresponding to (multiplicity of infection (MOI); that is, phage/bacterium ratio) of 0, 0.1, 1, 10 and 100 after 1 h of bacterial inoculation. However, to observe the effect of bacteriophage infection during the biofilm maturation stage, the bacteriophage(s) was added to 24-h-old-biofilms (the cell density was approximately 108 colony forming units per cm3) at 0, 107, 108, 109 and 1010 PFU ml−1, corresponding to MOI of 0, 0.1, 1, 10 and 100, respectively. The plates were then incubated for another 24 h before recording the optical density at 600 nm and processing the crystal violet-based biofilm formation assay as previously described (May et al., 2009). Results showed that the bacteriophage infection dramatically inhibited the early biofilm formation by E. coli carrying a natural F-plasmid (Figure 1a), but they failed to attack the 24-h-old mature biofilms (Figure 1b). Interestingly, the early biofilm formation was more affected by the non-lytic filamentous f1 phage than both the lytic MS2 phage alone and the phage combination (the mixture of f1 and MS2 phages). The f1 phage inhibited the biofilm formation significantly when added at the MOI of 0.1 or higher without interfering planktonic cell growth or causing cell death. Infection by either the MS2 phage or the phage combination slightly influenced the early biofilm formation and caused cell death at only MOI more than 10. When both phages were added together, it is possible that binding of MS2 phage to F-pili could prevent binding of the f1 phage to the tip of F-pili (Ou, 1973). As the F-plasmid generates F-pili connections by the F+ × F+ mating pairs at early biofilm developmental stages (May and Okabe, 2008, Pereira et al., 2010), the results in this study again confirmed that F-pili is an important factor for cell attachment and microcolony formation by E. coli carrying a natural repressed F-plasmid. This is very similar to the case of E. coli carrying the de-repressed F-plasmid (Ghigo, 2001; Reisner et al., 2003). Therefore, this may suggest that the tip of F-pili is the specific attachment site for the formation of cell–cell mating pairs during the early biofilm formation, and the side of F-pili has a non-obligatory role.
Effect of bacteriophage(s), f1 and MS2 (either alone or in combination), on planktonic growth and early or late biofilm formation by a natural F-plasmid carrying E. coli. Bacteriophage(s) (that is, at MOIs ranging from 0 to 100) were introduced during early bacterial attachment stage (1 h of inoculation) (a) or late biofilm maturation stage (24 h after biofilms were formed) (b). Planktonic growth was recorded by reading the optical density at 600 nm. Biofilm biomass was stained with crystal violet and dissolved with 80:20 ethanol:acetone solution before reading the optical density at 570 nm. Results are averages of eight replicates±s.d. and are representative of four independent experiments. Statistically significant values, when compared with their non-infected condition, are indicated with ‘asterisk’ or ‘double asterisk’ at P<0.05 or P<0.01, respectively. w/, with; w/o, without.
To obtain a clear picture whether the f1 phage immediately and completely inhibits the F-pili mating pairs, we used flow-cell chambers (Stovall Life Science, Greensboro, NC, USA) and monitored biofilm development under a LSM510 Confocal Laser Scanning Microscope (Carl Zeiss, Oberkochen, Germany) as previously described (May and Okabe, 2008). Therefore, E. coli carrying F-plasmid was genetically tagged with a DsRed-Express fluorescent protein (Clontech, Mountain View, CA, USA) and then inoculated into the flow-cells (grown on M9-glucose medium at 37 °C). The f1 phage (108 PFU ml−1) was directly injected into the flow-cells after the biofilms were grown for 2, 6, 12, 24 and 48 h, respectively (Figure 2a). After phage injection, the flow-cells were incubated for another 12 h in each condition, and then LSM projection images of biofilms were taken to quantify the biofilm morphology using a COMSTAT computer software (Heydorn et al., 2000) (Figure 2b). Furthermore, total RNA was harvested from the flow-cells to determine the mRNA expression level of the F-pili major subunit, traA, which was normalized with the expression level of ftsZ E. coli housekeeping gene, according to a protocol as previously described (May et al., 2010). The number of F-pili was also measured by directly counting for 100 transmitted electron microscope images and by applying the phage binding assay as previously described (Ou and Anderson, 1972; Ou, 1973) (Figure 2c). The results clearly showed that the f1 phage infection completely inhibited F-pili mediated bacterial attachment and microcolony formation, as indicated by less biomass, substratum coverage and average thickness, when the f1 phage was added at 2, 6 and 12 h, respectively (Figure 2b). However, no inhibitory effect was observed for the 24-h- and 48-h-old mature biofilms (the average thickness was at least 20 μm). These biofilms became slightly flatter after the f1 phage infection as indicated by smaller roughness coefficients (Figure 2b). This might be explained by the fact that the F-pili mating pairs are formed mainly outside the mature biofilms owing to higher reproductive activity (May et al., 2010), and thus the f1 phage could infect cells located in the outermost of the biofilm. Based on the level of traA transcripts as a measure of transcription of the entire conjugative-factor including the F-pili subunit synthesis (Frost and Manchak, 1998), we found that F-pili transcripts was at the maximum during the microcolony formation stage (6–12 h) and decreased during the late biofilm development and maturation stages (24–48 h) (Figure 2c). Similarly, the number of F-pili declined in the biofilm maturation stage (Figure 2c). Therefore, the F-pili production was dependent on the age of biofilms. It is therefore speculated that the regulation of F-pili synthesis during the biofilm formation might determine the infection capacity of male-specific bacteriophages.
The three-dimensional structure of non-infected and infected biofilms upon exposure to f1 phage at different biofilm ages, at 2, 6, 12, 24, or 24 h, respectively (a). After 108 PFU ml−1 of f1 phage infection, flow-cells were incubated for 12 h and the three-dimensional projection images were taken. The substratum coverage, average thickness and roughness coefficient of the biofilms were quantified using the COMSTAT computer software (b). The relative mRNA expression level of the F-pili subunit, traA, (that is, compared against an ftsZ housekeeping mRNA transcripts) was determined using the quantitative reverse transcription PCR (c). In all, 100 transmitted electron microscope photographs were used to examine the number of F-pili per 100 bacterial cells during biofilm formation (c). All experiments were duplicated, and representative images/values are shown. Statistically significant values, when compared with the non-infected condition, are indicated with ‘asterisk’ or ‘double asterisk’ at P<0.05 or P<0.01, respectively. n.d., unable to be detected (because of not enough attached cells on the surfaces).
Several mechanisms could be proposed to explain the failure of the f1 phage infection to E. coli mature biofilms, including the restricted penetration of bacteriophage into biofilms and emergence of biofilm-specific phenotype. E. coli carrying a natural conjugative F-plasmid can replace the F-pili mating pairs by inducing colonic acid (encoded by the cps genes) and curli (encoded by the csg genes), which promotes biofilm development and maturation (Da Re et al., 2007, May and Okabe, 2008). To investigate whether the biofilm-specific phenotype such as production of colonic acid and curli contributed to the failure of the f1 phage infection to E. coli mature biofilms, we examined this hypothesis using the ΔcpsE and ΔcsgA deletion mutants. Both strains were obtained from the National Institute of Genetics (Japan), and then the natural F-plasmid was inserted into the strains. Deletion of csgA abolished the ability to form thick biofilms owing to a lack of curli, and subsequently the ΔcsgA mature biofilms were impaired by the f1 phage infection (Figure 3). This suggests that production of curli fibers might have a role in resistance to the f1 phage infection. To confirm this speculation, the curli-overexpressed strain, PHL628 (Prigent-Combaret et al., 2000), carrying a natural F-plasmid was also tested for the f1 phage infection (Figure 3). This strain was originally provided by Professor Corinne Dorel of the INSA de Lyon, France. In addition, soluble curli fibers were measured using Thioflavin T (Sigma-Aldrich, St Louis, MO, USA), an amyloid-specific dye, which has been commonly used to assay curli formation (Wang et al., 2007). Overexpression of curli fibers promoted thick biofilm formation, which totally resisted the f1 phage infection. The mature biofilms of ΔcpsE mutant were intact after f1 phage infection, indicating that production of colonic acid was not directly contributed to the resistance to the f1 phage infection. Taken together, our observations indicate that the induction of curli fibers during biofilm development and maturation might be an advantage of E. coli carrying a natural repressed F-plasmid to prevent bacteriophages infection. The detailed mechanism is, however, not known presently. We are currently investigating the fate (that is, phage cycle and localization) of the filamentous f1 bacteriophage within mature biofilms, as well as the possibility that increased curli production is a part of coordinated responses to attack by bacteriophages.
Contribution of colonic acid and curli productions to the failure of bacteriophage infection in both planktonic cultures and within mature biofilms. The curli-deficient strain (ΔcsgA) and the curli-overexpressed strain (PHL628) and the colonic acid-deficient strain (ΔcpsE) were used. The 108 PFU ml−1 of f1 phage were added after biofilms were formed for 24 h. Soluble curli fibers were measured using Thioflavine T (TnT) assay. Results are averages of eight replicates ±s.d. and are representative of four independent experiments. Statistically significant values, when compared with their non-infected condition, are indicated with ‘asterisk’ or ‘double asterisk’ at P<0.05 or P<0.01, respectively. w/, with; w/o, without.
In conclusion, our results suggest that growth as a biofilm is an advantage for bacterial cells to prevent the male-specific bacteriophage infection because the formation of F-pili mating pairs declines and curli production already commences during biofilm development and maturation. This highlights a close relationship between the ability of bacteria carrying a conjugative plasmid to form a mature biofilm and the resistance to male-specific bacteriophages infection. Furthermore, filamentous male-specific bacteriophages are able to completely inhibit the initial bacterial attachment by E. coli carrying a conjugative plasmid. This result might pave the way for new treatments for biofilm-related infections and biofilms in general.
References
Blattner FR, Plunkett III G, Bloch CA, Perna NT, Burland V, Riley M et al. (1997). The complete genome sequence of Escherichia coli K-12. Science 277: 1453–1462.
Corbin BD, McLean RJC, Aron GM . (2001). Bacteriophage T4 multiplication in a glucose-limited Escherichia coli biofilm. Can J Microbiol 47: 680–684.
Da Re S, Le Quere B, Ghigo JM, Beloin C . (2007). Tight modulation of Escherichia coli bacterial biofilm formation through controlled expression of adhesion factors. Appl Environ Microbiol 73: 3391–3403.
Doolittle MM, Cooney JJ, Caldwell DE . (1995). Lytic infection of Escherichia coli biofilms by bacteriophage T4. Can J Microbiol 41: 12–18.
Doolittle MM, Cooney JJ, Caldwell DE . (1996). Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J Ind Microbiol Biotechnol 16: 331–341.
Frost LS, Ippen-Ihler K, Skurray RA . (1994). Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Mol Biol Rev 58: 162–210.
Frost LS, Manchak J . (1998). F− phenocopies: characterization of expression of the F transfer region in stationary phase. Microbiology 144: 2579–2587.
Garcia LR, Molineux IJ . (1995). Incomplete entry of bacteriophage T7 DNA into F plasmid-containing Escherichia coli. J Bacteriol 177: 4077–4083.
Ghigo JM . (2001). Natural conjugative plasmids induce bacterial biofilm development. Nature 412: 442–445.
Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK et al. (2000). Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146: 2395–2407.
Lacqua A, Wanner O, Colangelo T, Martinotti MG, Landini P . (2006). Emergence of biofilm-forming subpopulations upon exposure of Escherichia coli to environmental bacteriophages. Appl Environ Microbiol 72: 956–959.
May T, Ito A, Okabe S . (2009). Induction of multidrug resistance mechanism in Escherichia coli biofilms by interplay between tetracycline and ampicillin resistance genes. Antimicrob Agents Chemother 53: 4628–4639.
May T, Ito A, Okabe S . (2010). Characterization and global gene expression of F− phenocopies during Escherichia coli biofilm formation. Mol Genet Genomics doi:10.1007/s00438-010-0571-2.
May T, Okabe S . (2008). Escherichia coli harboring a natural IncF conjugative F plasmid develops complex mature biofilms by stimulating synthesis of colanic acid and curli. J Bacteriol 190: 7479–7490.
Ou JT, Anderson TF . (1972). Effect of Zn2+ on bacterial conjugation: inhibition of mating pair formation. J Bacteriol 111: 177–185.
Ou JT . (1973). Inhibition of formation of Escherichia coli mating pairs by f1 and MS2 bacteriophages as determined with a coulter counter. J Bacteriol 114: 1108–1115.
Pereira A, Silva T, Gomes A, Araujo A, Giugliano L . (2010). Diarrhea-associated biofilm formed by enteroaggregative Escherichia coli and aggregative Citrobacter freundii: a consortium mediated by putative F pili. BMC microbiol 10: 57.
Prigent-Combaret C, Prensier G, Le Thi TT, Vidal O, Lejeune P, Dorel C . (2000). Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colanic acid. Environ Microbiol 2: 450–464.
Reisner A, Haagensen JAJ, Schembri MA, Zechner EL, Molin S . (2003). Development and maturation of Escherichia coli K-12 biofilms. Mol Microbiol 48: 933–946.
Reisner A, Höller BM, Molin S, Zechner EL . (2006a). Synergistic effects in mixed Escherichia coli biofilms: conjugative plasmid transfer drives biofilm expansion. J Bacteriol 188: 3582–3588.
Reisner A, Krogfelt KA, Klein BM, Zechner EL, Molin S . (2006b). In vitro biofilm formation of commensal and pathogenic Escherichia coli strains: Impact of environmental and genetic factors. J Bacteriol 188: 3572–3581.
Wang X, Smith DR, Jones JW, Chapman MR . (2007). In vitro polymerization of a functional Escherichia coli amyloid protein. J Biol Chem 282: 3713–3719.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
May, T., Tsuruta, K. & Okabe, S. Exposure of conjugative plasmid carrying Escherichia coli biofilms to male-specific bacteriophages. ISME J 5, 771–775 (2011). https://doi.org/10.1038/ismej.2010.158
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2010.158
Keywords
This article is cited by
-
Phage co-transport with hyphal-riding bacteria fuels bacterial invasion in a water-unsaturated microbial model system
The ISME Journal (2022)
-
Phage mobility is a core determinant of phage–bacteria coexistence in biofilms
The ISME Journal (2018)
-
Dynamic biofilm architecture confers individual and collective mechanisms of viral protection
Nature Microbiology (2017)