Abstract
The lithistid sponge Discodermia dissoluta (family Theonellidae), is found in deep-waters throughout the Caribbean sea and is the source of discodermolide, a natural product with potential anticancer properties, and other secondary metabolites. As with other sponges, large numbers of microbes are harbored in the sponge mesohyl. The microbial population of the sponge mesohyl shows an abundance of large filamentous microbes. Fractionation of the dissociated sponge allowed enrichment of this microbe, which was then identified by analysis of the 16S rRNA genes. Its identity was confirmed through the use of fluorescent in situ hybridization. These studies have allowed the identification of this eubacterial microbe as belonging to the genus Entotheonella.
Similar content being viewed by others
Introduction
Sponges contribute significantly to the total fauna of sessile marine organisms worldwide and dominate the benthic community of some Caribbean and other tropical waters (Hentschel et al., 2002). Even though sponges feed on microorganisms, large numbers of bacteria are also known to be harbored within the extracellular mesohyl matrix of many sponges, living in symbiosis with their host (Imhoff and Stöhr, 2003). This microbial community has been shown to be distinct from that of marine plankton or marine sediments and therefore is specific to the sponge, rather than to its environment (Hentschel et al., 2006). Even though a comprehensive overview of microbial diversity from marine invertebrates can be achieved through various culture-dependent and culture-independent techniques (Fieseler et al., 2004; Lesser et al., 2004), a combination of microscopic methods such as transmission electron microscopy (TEM) and sequence-specific fluorescence in situ hybridization (FISH) may enable the precise localization of particular microbes within the invertebrate host.
In this study, we identify a filamentous microorganism in the lithistid sponge Discodermia sp. (family Theonellidae) with 16S rRNA gene analysis and localize it to the sponge mesohyl using TEM and FISH probing.
Materials and methods
Chemicals were purchased from Sigma (St Louis, MO, USA), unless otherwise stated. Primers and probes were purchased from MWG Biotech (High Point, NC, USA).
Samples of Discodermia sp. were collected at depths between 480 and 500 ft (146–152 m) around Grand Bahama Island (Bahamas), using the Research Vessels Seward Johnson I and Seward Johnson II and the Johnson-Sea-Link I submersible in October 2000 and March 2001.
Observations by TEM were made on 2 × 3 mm fragments of sponge tissue, fixed using a modified ‘low osmium pre-fixative’ technique as described previously (Eisenman and Alfert, 1981). In one series of samples, ruthenium red was added to each solution in the proportion of 50 mg per 100 ml (Luft, 1971a, 1971b) to enhance contrast of the cell coat. The samples were post-fixed for 3 h in 1% osmium tetroxide in 0.2 M sodium cacodylate and 0.3 M NaCl, dehydrated through a graded ethanol series, and embedded in ERL 4206 (Spurr, 1969).
The procedure for the separation of symbiotic bacteria from sponge cells was carried out as previously described (Bewley et al., 1996). The separation yielded a pellet (1P), highly enriched in filamentous bacteria, while still containing a small number of unicellular bacteria and sponge cells, and a supernatant (1S) containing a mixture of sponge cells and filamentous and unicellular bacteria.
1P samples were placed in a sterile mortar chilled with liquid nitrogen, in which the pellet was ground to a fine powder. The ground pellet was then transferred into a clean microcentrifuge tube, to which 500 μl of GTC buffer (4 M guanidine iso-thiocyanate, 25 mM sodium citrate, 0.5% SDS, pH 7.0) was added. DNA was extracted using a standard phenyl–chloroform–isoamylalcohol (24:1:1) protocol. The pellets were allowed to air dry before being resuspended in 50 μl of deionized H2O.
Two sets of universal bacterial primers (Table 1) were used to amplify ∼750 and ∼1300 bp of the 16S small subunit rRNA gene, using published protocols. Cleaned PCR reactions were shipped overnight to Northwoods DNA, Inc. (Solway, MN, USA), for sequencing. Edited sequences were queried using NCBI BLAST v 2.2 (Altschul et al., 1997).
FISH with Cy3 monolabeled (5′) rRNA-targeted probes was carried out using probes covering most bacteria (EUB388, Amann et al., 1990; EUBII, EUBIII, Daims et al., 1999) and a probe for most δ-proteobacteria (DELTA495suite, Loy et al., 2002). An Entotheonella sp. specific probe, designed using the ARB probe design tool and a control probe with one mismatch, was monolabeled (5′) with FAM to enable dual hybridization with DELTA495suite. All probes are listed in Table 1. Protocols were performed as described previously, using a 1:10 (w/v) suspension of sponge cells fixed in 96% (v/v) ethanol (Brück et al., 2007). Hybridization was carried out at 46 °C for both probes while washing was at 48 °C. Bacteria were evaluated using an Olympus IX51 microscope (Center Valley, PA, USA) with an epifluorescence attachment (Olympus U-RFL-T) and Cy3 and FAM filter sets. Image analysis was performed using an Olympus DP70 camera system and CellF imaging software.
All phylogenetic reconstruction for sequences obtained from bacterial culture was performed using ARB (Ludwig et al., 2004). De novo trees were constructed using the ARB neighbor joining distance matrix with Felsenstein correction and termini (.-=0) and position variance (123456789.-=0) filters. New sequences generated in this study are available in GenBank (DQ519075, DQ519076, EU159414).
Results
Transmission electron microscopy on thin sections of sponge tissue, confirmed the presence of the filamentous organism in the sponge mesohyl in addition to a large number of other unicellular microorganisms (Figures 1a and b). The organism was absent in choanocyte chambers and other areas of the sponge. After cell separation and DNA extraction, 16S rRNA-specific primers showed amplification of ∼750 and ∼900 bp bands in fractions containing the filamentous organism. The final edited sequences of 501, 602 and 875 bp were 96, 97 and 93%, similar to uncultured Entotheonella sp. (AY897125, AY897120) from a Discodermia sp. metagenomic library previously described in literature (Schirmer et al., 2005).
(a) Transmission electron microscopy of filamentous structures in the mesohyl of Discodermia sp. fixed in the presence of ruthenium red and treated with hydrofluoric acid prior to embedding (bar=1.7 μm); (b) TEM of sponge material showing fine structures fixed without ruthenium red and treated with hydrofluoric acid after embedding (bar=0.4 μm); (c) FISH image (× 1000 magnification) of Discodermia sp. sample enriched for filamentous structures and spiked with E. coli as fluorescence control (EUB338); (d) FISH image (× 1000 magnification) of Discodermia sp. sample enriched for filamentous structures and hybridized with DELTA495suite. Figures (c) and (d) were taken using identical gain and exposure settings, (e) FISH image (× 1000 magnification) Discodermia sp. sample enriched for filamentous structures and hybridized with ENP 1442; (f) FISH image (× 1000 magnification) Discodermia sp. sample enriched for filamentous structures and hybridized with a control probe ENP 1442c containing 1 mismatch to ENP1442.
FISH was performed using EUB338, EUB338II, EUB338III, DELTA495a, ENP1442 and ENP1442c. A control sample was also prepared without probes. EUB338 hybridized well with the filamentous structures contained in the enriched fraction and showed similar signal strength when compared to an Escherichia coli standard mixed with the hybridization mixes. EUB338II and EUB338III hybridized only weakly. A similar level of fluorescence was observed when no probe was used, suggesting that the signal was due to autofluorescence. When the enriched samples were hybridized to the probes targeting Entotheonella sp. and total δ-proteobacteria (ENP1442, DELTA495suite), strong hybridization was observed and the filamentous structures were illuminated by the probes showing that the sequence originated within those organisms (Figure 1d). The mismatch probe illuminated weakly at identical gain levels, suggesting autofluorescence. Dual hybridization with DELTA495suite and the Entotheonella sp. specific probe (ENP1442), resulted in organisms emitting a yellow signal in composite images (not shown).
Discussion
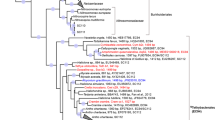
In this study, we identified the filamentous microorganism in the lithistid sponge Discodermia sp. as Entotheonella sp. through 16S rRNA gene analysis and FISH probing. The genus Entotheonella was first proposed by Schmidt et al. (2000) as a genus in the δ-subdivision of Proteobacteria, ‘Candidatus Entotheonella palauensis’ isolated from the Palauan marine sponge Theonella swinhoei. Another study, examining metagenomic libraries from total sponge tissue of D. dissoluta and enriched bacterial symbionts showed an abundance of Entotheonella sp. related sequences (Schirmer et al., 2005). However, due to the nature of the later study, no direct confirmation or no assumptions based on their location within D. dissuluta were made. We concluded that Entotheonella sp. is an abundant member of the symbiotic microbial community of the sponge mesohyl of Discodermia sp. As in T. swinhoei, where E. palauensis is thought to produce theopalauamide (Schmidt et al., 2000), all Entotheonella sp. found in this study were specific to the sponge mesohyl. TEM further showed that Entotheonella is not present in other areas of the sponge such as the choanocyte chambers where other unicellular microorganisms are present. This indicates a direct microbe–host association between Discodermia sp. and Entotheonella sp. instead of being a result of indiscriminate filter feeding (Figure 2).
Distance-based neighbor-joining phylogeny of 16S rRNA sequences, including putative Entotheonella sequences (WMB10, WMB11, WMB12). Sponge sources for Entotheonella sp. are given behind sequence names: (D) Discodermia sp., (T) T. swinhoei. Reference sequences are derived from ARB and are written with their corresponding accession numbers. Bootstrap percentages after 1000 replications for the most significantly supported clades are shown in bold below the nodes.
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402.
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA . (1990). Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56: 1919–1925.
Bewley CA, Holland ND, Faulkner DJ . (1996). Two classes of metabolites from Theonella swinhoei are localized in distinct populations of bacterial symbionts. Experientia 52: 716–722.
Brück TB, Brück WM, Santiago-Vázquez LZ, McCarthy PJ, Kerr RG . (2007). Diversity of the bacterial communities associated with the azooxanthellate deep water octocorals Leptogorgia minimata, Iciligorgia schrammi, and Swiftia exertia. Mar Biotechnol 9: 561–576.
Daims H, Brühl A, Amann R, Schleifer K-H, Wagner M . (1999). The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22: 434–444.
Eisenman EA, Alfert M . (1981). A new fixation procedure for preserving the ultrastructure of marine invertebrate tissues. J Microscopy 125: 117–120.
Fieseler L, Horn M, Wagner M, Hentschel U . (2004). Discovery of the novel candidate phylum ‘Poribacteria’ in marine sponges. Appl Env Microbiol 70: 3724–3732.
Hentschel U, Hopke J, Horn M, Friedrich AB, Wagner M, Hacker J et al. (2002). Molecular evidence for a uniform microbial community in sponges from different oceans. Appl Environ Microbiol 68: 4431–4440.
Hentschel U, Usher KM, Taylor MW . (2006). Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55 (2): 167–177.
Imhoff JF, Stöhr R . (2003). Sponge-associated bacteria: general overview and special aspects of bacteria associated with Halichondria panicea. Prog Mol Subcell Biol 37: 35–57.
Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR . (1985). Rapid determination of 16S ribosomal sequences for phylogenetic analysis. Proc Natl Acad Sci USA 82: 6955–6959.
Lesser MP, Mazel CH, Gorbunov MY, Falkowski GP . (2004). Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305: 997–1000.
Loy A, Lehner A, Lee N, Adamczyk J, Meier H, Ernst J et al. (2002). Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl Environ Microbiol 68: 5064–5081.
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371.
Luft JH . (1971a). Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec 171: 347–368.
Luft JH . (1971b). Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec 171: 369–416.
Schirmer A, Gadkari R, Reeves CD, Ibrahim F, DeLong EF, Hutchinson CR . (2005). Metagenomic analysis reveals diverse polyketide synthase gene clusters in microorganisms associated with the marine sponge Discodermia dissoluta. Appl Environ Microbiol 71: 4840–4849.
Schmidt EW, Obratsova AY, Davidson SK, Faulkner DJ, Haygood MG . (2000). Identification of the antifungal peptide-containing symbiont of the marine sponge Theonella swinhoei as a novel δ-proteobacterium, ‘Candidatus Entotheonella palauensis’. Mar Biol 136: 969–977.
Sfanos K, Harmody D, Dang P, Ledger A, Pomponi S, McCarthy PJ et al. (2005). A molecular systematic survey of cultured microbial associates of deep water marine invertebrates. Syst Appl Microbiol 28: 242–264.
Spurr AR . (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26: 31–43.
Acknowledgements
Sectioning for TEM was carried out at the Laboratoire de Biologie Marine, Université Libre de Bruxelles and observations were performed at the Laboratorium voor Entomologie, Departement Biologie, Katholieke Universiteit Leuven, Belgium. This research was funded through a postdoctoral fellowship to WMB from the Link Foundation. The experiments performed in this study comply with the current laws of the USA. This is HBOI contribution number 1686.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brück, W., Sennett, S., Pomponi, S. et al. Identification of the bacterial symbiont Entotheonella sp. in the mesohyl of the marine sponge Discodermia sp.. ISME J 2, 335–339 (2008). https://doi.org/10.1038/ismej.2007.91
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ismej.2007.91
Keywords
This article is cited by
-
Expressed protein profile of a Tectomicrobium and other microbial symbionts in the marine sponge Aplysina aerophoba as evidenced by metaproteomics
Scientific Reports (2018)
-
Co-occurring Mangroves and Salt Marshes Differ in Microbial Community Composition
Wetlands (2018)
-
Diversity of Actinobacteria Associated with the Marine Ascidian Eudistoma toealensis
Marine Biotechnology (2015)
-
An environmental bacterial taxon with a large and distinct metabolic repertoire
Nature (2014)
-
Phylogenetic diversity of culturable fungi associated with two marine sponges: Haliclona simulans and Gelliodes carnosa, collected from the Hainan Island coastal waters of the South China Sea
Fungal Diversity (2010)