Abstract
Background and objective:
The suckling period is a critical phase of development, in which maternal overnutrition may program the susceptibility of developing chronic diseases and disorders, such as obesity and metabolic alterations, in adult life. Here, we questioned whether the consumption of a cafeteria diet throughout lactation in rats affects the macronutrient composition of milk and whether it results in permanent metabolic effects in the offspring.
Methods:
Nursing rats were fed a control diet or a cafeteria diet during lactation. Milk was obtained at different time points of lactation. Offspring (males and females) were weaned onto a control diet until the age of 6 months. Circulating parameters were measured under ad libitum feeding and under 12-h fasting conditions at weaning and at 3 and 6 months of age. An oral glucose tolerance test (OGTT) was performed at 3 and 6 months of age.
Results:
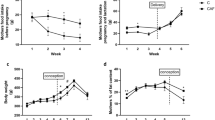
Rats fed a cafeteria diet during lactation consumed an unbalanced diet, with lower protein and higher fat content versus controls, which was reflected in the composition of the milk. The offspring of rats fed a cafeteria diet during lactation showed lower body weight and lower lean mass, but greater fat accumulation, compared with controls. They also displayed hyperleptinaemia, altered lipid profile and impaired response to an OGTT.
Conclusion:
Maternal consumption of a cafeteria diet throughout lactation in rats produces lasting effects in the metabolic health of their offspring, which are not associated with a higher body weight but with a greater fat accumulation, similarly to the thin-outside-fat-inside phenotype.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Langley-Evans SC . Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet 2015; 28 (Suppl 1): 1–14.
McMillen IC, Robinson JS . Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005; 85: 571–633.
Barker DJ, Eriksson JG, Forsen T, Osmond C . Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002; 31: 1235–1239.
Langley-Evans SC . Developmental programming of health and disease. Proc Nutr Soc 2006; 65: 97–105.
Martorell R, Stein AD, Schroeder DG . Early nutrition and later adiposity. J Nutr 2001; 131: 874S–880S.
Pico C, Palou M, Priego T, Sanchez J, Palou A . Metabolic programming of obesity by energy restriction during the perinatal period: different outcomes depending on gender and period, type and severity of restriction. Front Physiol 2012; 3: 436.
Akyol A, McMullen S, Langley-Evans SC . Glucose intolerance associated with early-life exposure to maternal cafeteria feeding is dependent upon post-weaning diet. Br J Nutr 2012; 107: 964–978.
Sun B, Purcell RH, Terrillion CE, Yan J, Moran TH, Tamashiro KL . Maternal high-fat diet during gestation or suckling differentially affects offspring leptin sensitivity and obesity. Diabetes 2012; 61: 2833–2841.
Bayol SA, Simbi BH, Bertrand JA, Stickland NC . Offspring from mothers fed a 'junk food' diet in pregnancy and lactation exhibit exacerbated adiposity that is more pronounced in females. J Physiol 2008; 586: 3219–3230.
Palou M, Konieczna J, Torrens JM, Sánchez J, Priego T, Fernandes ML et al. Impaired insulin and leptin sensitivity in the offspring of moderate caloric-restricted dams during gestation is early programmed. J Nutr Biochem 2012; 23: 1627–1639.
Palou M, Priego T, Sanchez J, Palou A, Pico C . Sexual dimorphism in the lasting effects of moderate caloric restriction during gestation on energy homeostasis in rats is related with fetal programming of insulin and leptin resistance. Nutr Metab (Lond) 2010; 7: 69.
Ainge H, Thompson C, Ozanne SE, Rooney KB . A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2011; 35: 325–335.
Armitage JA, Taylor PD, Poston L . Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol 2005; 565: 3–8.
Sánchez J, Priego T, García AP, Llopis M, Palou M, Picó C et al. Maternal supplementation with an excess of different fat sources during pregnancy and lactation differentially affects feeding behavior in offspring: putative role of the leptin system. Mol Nutr Food Res 2012; 56: 1715–1728.
Oben JA, Mouralidarane A, Samuelsson AM, Matthews PJ, Morgan ML, McKee C et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J Hepatol 2010; 52: 913–920.
Wright TM, Fone KC, Langley-Evans SC, Voigt JP . Exposure to maternal consumption of cafeteria diet during the lactation period programmes feeding behaviour in the rat. Int J Dev Neurosci 2011; 29: 785–793.
Del Prado M, Delgado G, Villalpando S . Maternal lipid intake during pregnancy and lactation alters milk composition and production and litter growth in rats. J Nutr 1997; 127: 458–462.
Priego T, Sanchez J, Garcia AP, Palou A, Pico C . Maternal dietary fat affects milk fatty acid profile and impacts on weight gain and thermogenic capacity of suckling rats. Lipids 2013; 48: 481–495.
Llopis M, Sánchez J, Priego T, Palou A, Picó C . Maternal fat supplementation during late pregnancy and lactation influences the development of hepatic steatosis in offspring depending on the fat source. J Agric Food Chem 2014; 62: 1590–1601.
Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity (Silver Spring) 2011; 19: 1109–1117.
Heyne A, Kiesselbach C, Sahún I, McDonald J, Gaiffi M, Dierssen M et al. An animal model of compulsive food-taking behaviour. Addict Biol 2009; 14: 373–383.
Pico C, Pons A, Gianotti M, Palou A . Sustained changes in blood alpha amino nitrogen compartmentation during recovery from cafeteria feeding in rats. Arch Int Physiol Biochim Biophys 1991; 99: 345–348.
Oliver P, Reynes B, Caimari A, Palou A . Peripheral blood mononuclear cells: a potential source of homeostatic imbalance markers associated with obesity development. Pflugers Arch 2013; 465: 459–468.
Castro H, Pomar CA, Picó C, Sánchez J, Palou A . Cafeteria diet overfeeding in young male rats impairs the adaptive response to fed/fasted conditions and increases adiposity independent of body weight. Int J Obes (Lond) 2014; 39: 430–437.
Rodríguez E, Ribot J, Rodríguez AM, Palou A . PPAR-gamma2 expression in response to cafeteria diet: gender- and depot-specific effects. Obes Res 2004; 12: 1455–1463.
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Martin Agnoux A, Antignac JP, Boquien CY, David A, Desnots E, Ferchaud-Roucher V et al. Perinatal protein restriction affects milk free amino acid and fatty acid profile in lactating rats: potential role on pup growth and metabolic status. J Nutr Biochem 2015; 26: 784–795.
Boxwell J, Ayson P, Ramenofsky M . Growth and metabolic parameters in pups of undernourished lactating rats. Physiol Behav 1995; 57: 469–475.
Remmers F, Schreuder MF, Gemke RJ . Delemarre-van de Waal HA. Energy intake and resting energy expenditure in adult male rats after early postnatal food restriction. Br J Nutr 2008; 99: 1149–1156.
Ozanne SE, Lewis R, Jennings BJ, Hales CN . Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci (Lond) 2004; 106: 141–145.
de Moura EG, Lisboa PC, Custódio CM, Nunes MT, de Picoli Souza K, Passos MC . Malnutrition during lactation changes growth hormone mRNA expression in offspring at weaning and in adulthood. J Nutr Biochem 2007; 18: 134–139.
Escobar-Morreale HF, Alvarez-Blasco F, Botella-Carretero JI, Luque-Ramírez M . The striking similarities in the metabolic associations of female androgen excess and male androgen deficiency. Hum Reprod 2014; 29: 2083–2091.
Loomba-Albrecht LA, Styne DM . Effect of puberty on body composition. Curr Opin Endocrinol Diabetes Obes 2009; 16: 10–15.
Thomas EL, Parkinson JR, Frost GS, Goldstone AP, Doré CJ, McCarthy JP et al. The missing risk: MRI and MRS phenotyping of abdominal adiposity and ectopic fat. Obesity (Silver Spring) 2012; 20: 76–87.
Díaz-Rúa R, García-Ruiz E, Caimari A, Palou A, Oliver P . Sustained exposure to diets with an unbalanced macronutrient proportion alters key genes involved in energy homeostasis and obesity-related metabolic parameters in rats. Food Funct 2014; 5: 3117–3131.
Díaz-Rúa R, van Schothorst EM, Keijer J, Palou A, Oliver P . Isocaloric high-fat feeding directs hepatic metabolism to handling of nutrient imbalance promoting liver fat deposition. Int J Obes (Lond) 2016; 40: 1250–1259.
Cao L, Liu X, Cao H, Lv Q, Tong N . Modified high-sucrose diet-induced abdominally obese and normal-weight rats developed high plasma free fatty acid and insulin resistance. Oxid Med Cell Longev 2012; 2012: 374346.
Acknowledgements
This research was supported by grants from the Spanish Government (AGL2012-33692; AGL2015-67019-P), and the Instituto de Salud Carlos III, Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y Nutrición, CIBERobn. Our laboratory is a member of the European Research Network of Excellence NuGO (The European Nutrigenomics Organization, EU Contract: no. FP6-506360).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pomar, C., van Nes, R., Sánchez, J. et al. Maternal consumption of a cafeteria diet during lactation in rats leads the offspring to a thin-outside-fat-inside phenotype. Int J Obes 41, 1279–1287 (2017). https://doi.org/10.1038/ijo.2017.42
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.42
This article is cited by
-
Mothers’ cafeteria diet induced sex-specific changes in fat content, metabolic profiles, and inflammation outcomes in rat offspring
Scientific Reports (2021)
-
Exposure to maternal obesity during suckling outweighs in utero exposure in programming for post-weaning adiposity and insulin resistance in rats
Scientific Reports (2019)
-
Maternal overnutrition by hypercaloric diets programs hypothalamic mitochondrial fusion and metabolic dysfunction in rat male offspring
Nutrition & Metabolism (2018)