Abstract
Background/Objectives:
Characterisation of the adipocyte cellular lineage is required for a better understanding of white adipose tissue homoeostasis and expansion. Although several studies have focused on the phenotype of the most immature adipocyte progenitors, very few tools exist to identify committed cells. In haematopoiesis, the CD38 ectoenzyme is largely used to delineate various stages of stem cell lineage commitment. We hypothesise that this marker could be used to identify committed preadipocytes.
Methods:
Complementary strategies including flow cytometry, cell-sorting approaches, immunohistochemistry and primary cultures of murine adipose progenitors isolated from different fat pads of control or high-fat diet exposed C57BL/6 J mice were used to determine the molecular expression profile, proliferative and differentiation potentials of adipose progenitors expressing the CD38 molecule.
Results:
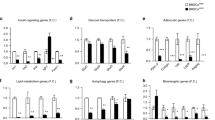
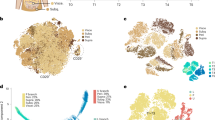
We demonstrate here that a subpopulation of CD45− CD31− CD34+ adipose progenitors express the cell surface protein CD38. Using a cell-sorting approach, we found that native CD45− CD31− CD34+ CD38+ (CD38+) adipose cells expressed lower CD34 mRNA and protein levels and higher levels of adipogenic genes such as Pparg, aP2, Lpl and Cd36 than did the CD45− CD31− CD34+ CD38− (CD38−) population. When cultivated, CD38+ cells displayed reduced proliferative potential, assessed by BrdU incorporation and colony-forming unit assays, and greater adipogenic potential. In vitro, both CD38 mRNA and protein levels were increased during adipogenesis and CD38− cells converted into CD38+ cells when committed to the adipogenic differentiation programme. We also found that obesity development was associated with an increase in the number of CD38+ adipose progenitors, this effect being more pronounced in intra-abdominal than in subcutaneous fat, suggesting a higher rate of adipocyte commitment in visceral depots.
Conclusions:
Together, these data demonstrate that CD38 represents a new marker that identifies committed preadipocytes as CD45− CD31− CD34low CD38+ cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rosen ED, Spiegelman BM . What we talk about when we talk about fat. Cell 2014; 156: 20–44.
Kajimura S, Spiegelman BM, Seale P . Brown and beige fat: physiological roles beyond heat generation. Cell Metab 2015; 22: 546–559.
Jeanson Y, Carriere A, Casteilla L . A new role for browning as a redox and stress adaptive mechanism? Front Endocrinol (Lausanne) 2015; 6: 158.
Rosenwald M, Perdikari A, Rulicke T, Wolfrum C . Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667.
Smorlesi A, Frontini A, Giordano A, Cinti S . The adipose organ: white-brown adipocyte plasticity and metabolic inflammation. Obes Rev 2012; 13 (Suppl 2): 83–96.
Cousin B, Bascands-Viguerie N, Kassis N, Nibbelink M, Ambid L, Casteilla L et al. Cellular changes during cold acclimatation in adipose tissues. J Cell Physiol 1996; 167: 285–289.
Lee YH, Petkova AP, Konkar AA, Granneman JG . Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 2015; 29: 286–299.
Wang QA, Tao C, Gupta RK, Scherer PE . Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19: 1338–1344.
Lee YH, Petkova AP, Mottillo EP, Granneman JG . In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15: 480–491.
Berry DC, Jiang Y, Graff JM . Emerging roles of adipose progenitor cells in tissue development, homeostasis, expansion and thermogenesis. Trends Endocrinol Metab 2016; 27: 574–585.
Jiang Y, Berry DC, Tang W, Graff JM . Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep 2014; 9: 1007–1022.
Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 2014; 19: 810–820.
Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB et al. Pdgfrbeta+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 2016; 23: 350–359.
Berry DC, Jiang Y, Graff JM . Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat Commun 2016; 7: 10184.
Pevsner-Fischer M, Levin S, Zipori D . The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev 2011; 7: 560–568.
Phinney DG . Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem 2012; 113: 2806–2812.
Baer PC . Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype In vivo and in vitro. World J Stem Cells 2014; 6: 256–265.
Berry R, Rodeheffer MS . Characterization of the adipocyte cellular lineage In vivo. Nat Cell Biol 2013; 15: 302–308.
Rodeheffer MS, Birsoy K, Friedman JM . Identification of white adipocyte progenitor cells In vivo. Cell 2008; 135: 240–249.
Maumus M, Peyrafitte JA, D'Angelo R, Fournier-Wirth C, Bouloumie A, Casteilla L et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (Lond) 2011; 35: 1141–1153.
Sengenes C, Lolmede K, Zakaroff-Girard A, Busse R, Bouloumie A . Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J Cell Physiol 2005; 205: 114–122.
Casteilla L, Planat-Benard V, Bourin P, Laharrague P, Cousin B . [Use of adipose tissue in regenerative medicine]. Transfus Clin Biol 2011; 18: 124–128.
Xiao M, Dooley DC . Cellular and molecular aspects of human CD34+ CD38- precursors: analysis of a primitive hematopoietic population. Leuk Lymph 2000; 38: 489–497.
Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008; 88: 841–886.
Song EK, Lee YR, Kim YR, Yeom JH, Yoo CH, Kim HK et al. NAADP mediates insulin-stimulated glucose uptake and insulin sensitization by PPARgamma in adipocytes. Cell Rep 2012; 2: 1607–1619.
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 2004; 109: 656–663.
Dromard C, Bourin P, Andre M, De Barros S, Casteilla L, Planat-Benard V . Human adipose derived stroma/stem cells grow in serum-free medium as floating spheres. Exp Cell Res 2011; 317: 770–780.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Berry R, Jeffery E, Rodeheffer MS . Weighing in on adipocyte precursors. Cell Metab 2014; 19: 8–20.
Barbosa MT, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P et al. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J 2007; 21: 3629–3639.
Goldberg LR, Dooner MS, Deng YH, Papa E, Pereira M, DelTatto M et al. Defining engraftment potential within the lineage positive population in murine marrow. Blood 2014; 124: 21.
Wiernik P, Goldman J, Dutcher J, Kyle R . Neoplastic diseases of the blood. Springer Science & Business Media 2012; 439: 203–206.
Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab 2012; 15: 838–847.
Drew JE, Farquharson AJ, Horgan GW, Williams LM . Tissue-specific regulation of sirtuin and nicotinamide adenine dinucleotide biosynthetic pathways identified in C57Bl/6 mice in response to high-fat feeding. J Nutr Biochem 2016; 37: 20–29.
Sanjabi B, Dashty M, Ozcan B, Akbarkhanzadeh V, Rahimi M, Vinciguerra M et al. Lipid droplets hypertrophy: a crucial determining factor in insulin regulation by adipocytes. Sci Rep 2015; 5: 8816.
Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS . Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol 2015; 17: 376–385.
Acknowledgements
We thank Dr Adriouch (Faculté de Médecine et de Pharmacie, Inserm U905, Institut de Recherche et d'Innovation Biomédicale, Université de Rouen, France), Dr Lund (Charles H. McCauley Professor and Chair Department of Microbiology, University of Alabama, Birmingham, United States) and Dr Pisani and Amri (Institut de Biologie Valrose, CNRS UMR7277, Inserm U1091, UNS Université Nice Sophia Antipolis, France) for fruitful discussions. We are grateful to the I2MC/UMR1048, GeT (Génome et Transcriptome) Platform, Génopole Toulouse. We also thank Dr D'Angelo and Zanoun for assistance and advice with imaging (Cellular Imaging Facility Rangueil-I2MC/TRI Platform). The authors thank the US006/CREFRE INSERM/UPS (Toulouse, France) and specifically the zootechnical core facility for animal care and the Toulouse RIO Imaging. We thank Mrs Zakaroff-Girard and Riant (Cytometry Core Facility, Inserm U1048, part of TRI Imaging Platform, Genotoul) for cell-sorting technical assistance. We especially thank Mrs Ducos for excellent technical assistance as well as Mrs Renoud, Achard, André and Mr De Vecchi (STROMALab) for their help in experiment management. This work was supported by the European Union Framework Programme 7 projects DIABAT [grant number HEALTH-F2-2011-278373] and METABOSTEM [grant number PCIG9-GA-2011-293720].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Carrière, A., Jeanson, Y., Côté, JA. et al. Identification of the ectoenzyme CD38 as a marker of committed preadipocytes. Int J Obes 41, 1539–1546 (2017). https://doi.org/10.1038/ijo.2017.140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2017.140