Abstract
Background/Objectives:
Prader–Willi syndrome (PWS) is a type of human genetic obesity that may give us information regarding the physiology of non-syndromic obesity. The objective of this study was to investigate the functional correlates of hunger and satiety in individuals with PWS in comparison with healthy controls with obesity, hypothesizing that we would see significant differences in activation in the left dorsolateral prefrontal cortex (DLPFC) based on prior findings.
Subjects/Methods:
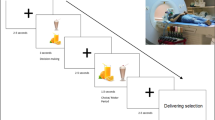
This study compared the central effects of food consumption in nine individuals with PWS (7 men, 2 women; body fat 35.3±10.0%) and seven controls (7 men; body fat 28.8±7.6%), matched for percentage body fat. H215O-PET (positron emission tomography) scans were performed before and after consumption of a standardized liquid meal to obtain quantitative measures of regional cerebral blood flow (rCBF), a marker of neuronal activity.
Results:
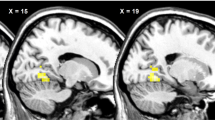
Compared with obese controls, PWS showed altered (P<0.05 family-wise error cluster-level corrected; voxelwise P<0.001) rCBF before and after meal consumption in multiple brain regions. There was a significant differential rCBF response within the left DLPFC after meal ingestion with decreases in DLPFC rCBF in PWS; in controls, DLPFC rCBF tended to remain unchanged. In more liberal analyses (P<0.05 family-wise error cluster-level corrected; voxelwise P<0.005), rCBF of the right orbitofrontal cortex (OFC) increased in PWS and decreased in controls. In PWS, ΔrCBF of the right OFC was associated with changes in appetite ratings.
Conclusions:
The pathophysiology of eating behavior in PWS is characterized by a paradoxical meal-induced deactivation of the left DLPFC and activation in the right OFC, brain regions implicated in the central regulation of eating behavior.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Whittington JE, Holland AJ, Webb T, Butler J, Clarke D, Boer H . Population prevalence and estimated birth incidence and mortality rate for people with Prader–Willi syndrome in one UK Health Region. J Med Genet 2001; 38: 792–798.
Holm VA, Cassidy SB, Butler MG, Hanchett JM, Greenswag LR, Whitman BY et al. Prader–Willi syndrome: consensus diagnostic criteria. Pediatrics 1993; 91: 398–402.
Zipf WB, Berntson GG . Characteristics of abnormal food-intake patterns in children with Prader–Willi syndrome and study of effects of naloxone. Am J Clin Nutr 1987; 46: 277–281.
Hinton EC, Holland AJ, Gellatly MSN, Soni S, Patterson M, Ghatei MA et al. Neural representations of hunger and satiety in Prader–Willi syndrome. Int J Obes Relat Metab Disord 2005; 30: 313–321.
Miller JL, Couch JA, Schmalfuss I, He G, Liu Y, Driscoll DJ . Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader–Willi syndrome. Am J Med Genet 2007; 143A: 476–483.
Iughetti L, Bosio L, Corrias A, Gargantini L, Ragusa L, Livieri C et al. Pituitary height and neuroradiological alterations in patients with Prader–Labhart–Willi syndrome. Eur J Pediatr 2008; 167: 701–702.
Shapira NA, Lessig MC, He AG, James GA, Driscoll DJ, Liu Y . Satiety dysfunction in Prader–Willi syndrome demonstrated by fMRI. J Neurol Neurosurg Psychiatry 2005; 76: 260–262.
Lucignani G, Panzacchi A, Bosio L, Moresco RM, Ravasi L, Coppa I et al. GABAA receptor abnormalities in Prader–Willi syndrome assessed with positron emission tomography and [11C]flumazenil. NeuroImage 2004; 22: 22–28.
Tataranni PA, Gautier J-F, Chen K, Uecker A, Bandy D, Salbe AD et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 1999; 96: 4569–4574.
Del Parigi A, Gautier J-F, Chen K, Salbe AD, Ravussin E, Reiman E et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann NY Acad Sci 2002; 967: 389–397.
Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG . A general mechanism for perceptual decision-making in the human brain. Nature 2004; 431: 859–862.
Le DSNT, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr 2006; 84: 725–731.
DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007; 31: 440–448.
Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader–Willi syndrome vs simple obesity. Int J Obes 2012; 36: 638–647.
WHO Study Group. Diabetes mellitus. WHO Tech Rep Ser 1985; 727: 11.
Ferraro R, Boyce VL, Swinburn B, De Gregorio M, Ravussin E . Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr 1991; 53: 1368–1371.
Tataranni PA, Ravussin E . Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr 1995; 62: 730–734.
Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ . Spatial registration and normalization of images. Hum Brain Mapp 1995; 3: 165–189.
Ashburner J, Friston K . Multimodal image coregistration and partitioning—a unified framework. NeuroImage 1997; 6: 209–217.
Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC . Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994; 1: 210–220.
Le DSN, Pannacciulli N, Chen K, Salbe AD, Del Parigi A, Hill JO et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr 2007; 86: 573–579.
Gautier J-F, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE et al. Effect of satiation on brain activity in obese and lean women. Obesity Res 2001; 9: 676–684.
Alonso-Alonso M, Pascual-Leone A . The right brain hypothesis for obesity. JAMA 2007; 297: 1819–1822.
Alonso-Alonso, Pascual-Leone A . THe right brain hypothesis for obesity. JAMA 2007; 297: 1819–1822.
Brooks SJ, Cedernaes J, Schiöth HB . Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 2013; 8: e60393.
Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL et al. Prefrontal–striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci USA 2010; 107: 14811–14816.
Zhang Y, Zhao H, Qiu S, Tian J, Wen X, Miller JL et al. Altered functional brain networks in Prader–Willi syndrome. NMR Biomed 2013; 26: 622–629.
Kringelbach ML, Rolls ET . The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004; 72: 341–372.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–2356.
Maddock RJ . The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci 1999; 22: 310–316.
Dimitropoulos A, Tkach J, Ho A, Kennedy J . Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012; 58: 303–312.
Frank S, Kullmann S, Veit R . Food related processes in the insular cortex. Front Hum Neurosci 2013; 7: 499.
Craig AD . How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3: 655–666.
Weston CSE. Another major function of the anterior cingulate cortex: the representation of requirements. Neurosci Biobehav Rev 2012; 36: 90–110.
Bush G, Luu P, Posner MI . Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci (Regul Ed) 2000; 4: 215–222.
Balleine BW, Delgado MR, Hikosaka O . The role of the dorsal striatum in reward and decision-making. J Neurosci 2007; 27: 8161–8165.
Boggio PS, Macedo EC, de Schwartzman JS, Brunoni D, Teixeira MCTV, Fregni F . Transcranial direct current stimulation: a novel approach to control hyperphagia in Prader–Willi syndrome. J Child Neurol 2009; 24: 642–643.
Bravo GL, Poje AB, Perissinotti I, Marcondes BF, Villamar MF, Manzardo AM et al. Transcranial direct current stimulation reduces food-craving and measures of hyperphagia behavior in participants with Prader–Willi syndrome. Am J Med Genet B 2016; 171: 266–275.
Alonso-Alonso M . Translating tDCS into the field of obesity: mechanism-driven approaches. Front Hum Neurosci 2013; 7: 512.
Gluck ME, Alonso-Alonso M, Piaggi P, Weise CM, Jumpertz-von Schwartzenberg R, Reinhardt M et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring, MD) 2015; 23: 2149–2156.
Goldstone AP, Holland AJ, Hauffa BP, Hokken-Koelega AC, Tauber M . Recommendations for the diagnosis and management of Prader–Willi syndrome. J Clin Endocrinol Metab 2008; 93: 4183–4197.
Acknowledgements
We thank the dietary, nursing and technical staff of the National Institutes of Health Clinical Unit in Phoenix, AZ, for their assistance. Most of all, the we thank the volunteers for their participation in the study. This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Author contributions
MR and CMW wrote the manuscript and analyzed the data. KC and PT helped analyzing the data. MGH analyzed parts of the data. ADP was principally involved in designing the study and collected the data. MR, ADP, KC, EMR, PT, JK, MGH, DSNTL and CMW contributed to the interpretations of findings and commented on and edited the drafts. JK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Reinhardt, M., Parigi, A., Chen, K. et al. Deactivation of the left dorsolateral prefrontal cortex in Prader–Willi syndrome after meal consumption. Int J Obes 40, 1360–1368 (2016). https://doi.org/10.1038/ijo.2016.75
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.75
This article is cited by
-
Obesity, Appetite, and the Prefrontal Cortex
Current Obesity Reports (2017)