Abstract
Background:
Immune activation contributes to the persistent state of inflammation associated with metabolic dysfunction in obesity. The specific immune receptors that sense metabolic stress signals and trigger inflammation are nevertheless largely unknown, and little is known on inflammatory and immune gene regulation in obesity.
Methods:
The study includes a cross-sectional and a longitudinal arm. Forty children and adolescents were enrolled: 22 obese subjects and 18 age-matched normal weight controls. Obese subjects participated in an 18-month therapeutic protocol, based on intensive lifestyle modification (dietary regimen, physical activity and behavioral interventions). Expression of genes involved in the inflammasome pathway, plasma concentration of the inflammasome-associated pro-inflammatory cytokines (interleukin (IL)-1β and IL-18) and indexes of microbial translocation (lipopolysaccharide (LPS), soluble CD14 (sCD14) and intestinal fatty acid-binding protein) were analyzed at baseline in obese subjects compared with controls, and after 18 months in obese subjects.
Results:
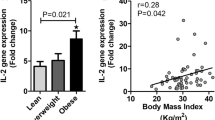
Cross-sectional analyses showed that the LPS-induced expression of genes involved in inflammasome (NLRP3, caspase 5 and NAIP), Nod-like receptors (NLRX1 and NOD1), downstream signaling (P2RX7, RAGE, RIPk2, TIRAP and BIRC2) and effector molecules (IFN-γ, IL-12β, IL-1β, CCL2, CCL5, IL-6 and TNFα) was significantly increased in obese subjects at baseline as compared with normal weight controls. The baseline plasma concentration of inflammasome-related cytokines (IL-1β and IL-18) and of microbial translocation markers (LPS and sCD14) was augmented in obese subjects as compared with controls as well. Longitudinal analyses indicated that intensive lifestyle modification resulted in a normalization of parameters in subjects with a significant reduction of BMI after 18 months.
Conclusions:
In children and adolescents, obesity is characterized by the activation of the inflammasome and by an alteration of gut permeability. Successful lifestyle modification is effective in reducing inflammation, suggesting that inhibition of the inflammasome may be a potential therapeutic strategy in obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
WHO Obesity and Overweight. Fact Sheet N° 311. WHO: Geneva, Switzerland., 2012.
De Onis M, Blossner M, Borghi E . Global prevalence and trends of overweight and obesity among preschool children. AM J Clin Nutr 2010; 92: 1257–1264.
Ogden CL, Carrol MD, Kit BK . Prevalence of childhood and adult obesity in the United States, 2011-2012. J Am Med Assn 2014; 311: 806–814.
Bleich S, Cutler D, Murray C, Adams A . Why is the developed world obese? Annu Rev Public Health 2008; 29: 273–295.
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH . Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997; 337: 869–873.
Allison DB, Fontaine KR, Manson JE, Stevens J, VaItallie TB . Annual deaths attributable to obesity in the United States. JAMA 1999; 282: 1530–1538.
Peirson L, Fitzpatrick-Lewis D, Morrison K, Warren R, Usman Ali M, Raina P . Treatment of overweight and obesity in children and youth: a systematic review and meta-analysis. CMAJ Open 2015; 3: E35–E46.
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I . Dietary gut microbial metabolites, short-chain fatty acids and host metabolic regulation. Nutrients 2015; 7: 2839–2849.
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP, Li HB . Impact of gut bacteria on human health and diseases. Int J Mol Sci 2015; 16: 7493–7519.
Everard A, Cani PD . Diabetes, obesity and gut microbiota. Best Prac Res Clin Gastr 2013; 27: 73–83.
Gregor MF, Hotamisligil GS . Inflammatory mechanisms in obesity. Annu Rev Immunol 2011; 29: 415–445.
Haneklaus M, O’Neill LA . NLRP3 at the interface of metabolism and inflammation. Immunol Rev 2015; 265: 53–62.
McNelis JC, Olefsky JM . Macrophages, immunity, and metabolic disease. Immunity 2014; 41: 36–48.
Odegaard JI, Chawla A . Mechanisms of macrophage activation in obesity-induced insulin resistance. Nat Clin Pract Endocrinol Metab 2008; 4: 619–622.
Olefsky JM, Glass CK . Macrophage, inflammation, ad insulin resistance. Annu Rev Physiol 2010; 72: 219–246.
Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Naver A et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells That affects metabolic parameters. Nat Med 2009; 15: 930–939.
Snyder-Cappione JE, Nikolajczyk BS . When diet and exercise are not enough, think immunomodulation. Mol Asp Of Med 2013; 34: 30–38.
Harrington DM, Staiano AE, Broyles ST, Gupta AK, Katzmarzyk PT . BMI percentiles for the identification of abdominal obesity and metabolic risk in children and adolescents: evidence in support of the CDC 95° percentile. Eur J Clin Nutr 2013; 67: 218–222.
Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 2006; 29: 581–593.
Edmunds L, Waters E, Elliott EJ . Evidence based management of childhood obesity. BMJ 2001; 323: 916–919.
National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents. Pediatric 2004; 114: 555–576.
Lohman TG, Roche AF, Martorell R . Anthropometrical Standardization Reference Manual Edited by Lohman TG, Roche AF, Martorell R Human Kinetics Books’: Champaign, IL, USA, 1988.
McCarthy HD, Jarrett KV, Crawley HF . The development of waist circumference percentiles in British children aged 5.0–16.9 y. Eur J Clin Nutr 2001; 55: 902–907.
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011; 128 (Suppl 5): S213–S256.
Epstein LH, Valoski A, Wing RR, McCurley J . Ten-year outcomes of behavioral family-based treatment for childhood obesity. Health Psychol 1994; 13: 373–383.
Epstein LH, Valoski A, Wing RR, McCurley J . Ten-year follow-up of behavioral, family-based treatment for obese children. JAMA 1990; 264: 2519–2523.
Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics 2007; 120 (Suppl 4): S254–S288.
Strasburger VC . American Academy of Pediatrics. Council on communications and media. Policy statement–children, adolescents, substance abuse, and the media. Pediatrics 2010; 126: 791–799.
Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y et al. Interventions for preventing obesity in children. Cochrane Database Syst Rev 2011; 12: CD001871.
American Academy of Pediatrics. Physical fitness and activity in schools. Pediatrics 2000; 105: 1156–1157.
Kissebah AH, Krakower GR . Regional adiposity and morbidity. Physiol Rev 1994; 74: 761–811.
Fonvig CE, Bille DS, Chabanova E, Nielsen TR, Thomsen HS, Holm JC . Muscle fat content and abdominal adipose tissue distribution investigated by magnetic resonance spectroscopy and imaging in obese children and youths. Pediatr Rep 2012; 4: e11.
Pedersen BK . Muscles and their myokines. J Exp Biol 2011; 214: 337–346.
I’Allemand D, Wiegand S, Reinehr T, Muller J, Wabitsch M, Widhalm K et al. Cardiovascular risk in 2008 European overweight children as established by a multicenter database. Obesity 2008; 16: 1672–1679.
Cole TJ, Lobstein T . Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012; 7: 284–294.
Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab 2011; 96: E146–E150.
Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE et al. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008; 57: 437–443.
Qi L, Rimm E, Liu S, Rifai N, Hu FB . Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 2005; 28: 1022–1028.
Pedersen BK, Fischer CP . Beneficial health effects of exercise-the role of IL-6 as a myokine. Trends Pharmacol Sci 2007; 28: 152–156.
Petersen AM, Pedersen BK . The anti-inflammatory effect of exercise. J Appl Physiol 2005; 98: 1154–1162.
Sims JE, Smith DE . The IL-1 family: regulators of immunity. Nat Rev Immunol 2010; 10: 89–102.
Dinarello CA . Interleukin-18 and the pathogenesis of inflammatory diseases. Semin Nephrol 2007; 27: 98–114.
Dinarello CA . The interleukin-1 family: 10 years of discovery. FASEB J 1994; 8: 1314–1325.
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H . Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol 2001; 19: 423–474.
Fowler BJ, Gelfand BD, Kim Y, Kerur N, Tarallo V, Hirano Y et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014; 346: 1000–1003.
American Diabetes Association. Standards of medical care in diabetes-2011. Diabetes Care 2011; 34 (Suppl 1): S11–S61.
Acknowledgements
This work was supported by Istituto Superiore di Sanità ‘Programma Nazionale di Ricerca sull’AIDS’; Ricerca Finalizzata and Ricerca Corrente (Italian Ministry of Health) and by King Saud University, Deanship of Scientific Research, Prince Mutaib Bin Abdullah Chair for Biomarkers of Osteoporosis.
Author contributions
VR, IS and MB performed immunological analyses and contributed in result interpretation. LS, EG and GVZ were responsible for the enrollment and the clinical management of the patients. CR performed statistical analyses. NMA-D contributed to the interpretation of the results and to writing the manuscript. VR, MC and DT designed, executed and interpreted the study, and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Rainone, V., Schneider, L., Saulle, I. et al. Upregulation of inflammasome activity and increased gut permeability are associated with obesity in children and adolescents. Int J Obes 40, 1026–1033 (2016). https://doi.org/10.1038/ijo.2016.26
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.26
This article is cited by
-
Effects of probiotic administration on overweight or obese children: a meta-analysis and systematic review
Journal of Translational Medicine (2023)
-
Eco-Evolutionary Dynamics of the Human-Gut Microbiota Symbiosis in a Changing Nutritional Environment
Evolutionary Biology (2022)
-
Roux-en-Y gastric bypass potentially improved intestinal permeability by regulating gut innate immunity in diet-induced obese mice
Scientific Reports (2021)
-
Intestinal barrier function in morbid obesity: results of a prospective study on the effect of sleeve gastrectomy
International Journal of Obesity (2020)
-
The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging
Biogerontology (2019)