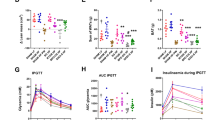
Abstract
Background/Objectives:
The combination of energy dense diets and reduced energy expenditure in modern society has escalated the prevalence of obesity and obesity-related comorbidities. Among these disease states, type-2 diabetics (T2D) are disproportionately associated with obesity, suggesting a shared etiology. In conjunction with defects in hormonal and inflammatory states, obesity and T2D are also characterized by dysbiosis.
Methods:
We have recently described the beneficial effects of duodenal nutrient exclusion, as induced by the duodenal endoluminal sleeve (DES); including body weight loss, prevented fat mass accumulation, and improved glucose tolerance in the ZDF rat, a rodent model of obesity and type-2 diabetes (T2D). To assess the relative role of DES on hindgut microbiota in the context of these metabolic changes, we analyzed cecal samples from rats implanted with a duodenal endoluminal sleeve (DES), or a sham control of this procedure. A group of pair-fed (pf) sham controls was also included to account for changes induced by reduced body weight and food intake.
Results:
Analysis of hindgut microbiota following DES in the ZDF rat elucidated discrete changes in several microbial populations including a reduction in Paraprevotella family members of the Clostridiales order along with an increase in Akkermansia muciniphila and species of the Allobaculum and Bifidobacterium genera.
Conclusions:
Altogether, these observations suggest that like Roux-en Y gastric bypass (RYGB) and Metformin, regulation of gut microbiota may be a contributing factor to the therapeutic effects of DES.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Smith KB, Smith MS . Obesity Statistics. Prim Care 2016; 43: 121–135.
Anhe FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D et al. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr Obes Rep 2015; 4: 389–400.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012; 490: 55–60.
Dore J, Simren M, Buttle L, Guarner F . Hot topics in gut microbiota. United European Gastroenterol J 2013; 1: 311–318.
Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N . Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front Microbiol 2016; 7: 185.
Liou AP, Paziuk M, Luevano Jr JM, Machineni S, Turnbaugh PJ, Kaplan LM . Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra41.
Lee H, Ko G . Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol 2014; 80: 5935–5943.
Habegger KM, Al-Massadi O, Heppner KM, Myronovych A, Holland J, Berger J et al. Duodenal nutrient exclusion improves metabolic syndrome and stimulates villus hyperplasia. Gut 2014; 63: 1238–1246.
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010; 59: 3049–3057.
Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013; 98: 16–24.
Kumar R, Maynard CL, Eipers P, Goldsmith KT, Ptacek T, Grubbs JA et al. Colonization potential to reconstitute a microbe community in patients detected early after fecal microbe transplant for recurrent C. difficile. BMC Microbiol 2016; 16: 5.
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD . Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and environmental microbiology 2013; 79: 5112–5120.
Kumar R, Eipers P, Little RB, Crowley M, Crossman DK, Lefkowitz EJ et al. Getting started with microbiome analysis: sample acquisition to bioinformatics. Curr Protoc Hum Genet 2014; 82: 18 8 1–18 829.
FASTQC. Available at http://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
FASTX-Toolkit. Available at http://hannonlab.cshl.edu/fastx_toolkit/.
Edgar RC . Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26: 2460–2461.
Wang Q, Garrity GM, Tiedje JM, Cole JR . Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007; 73: 5261–5267.
McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 2012; 6: 610–618.
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R . PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 2010; 26: 266–267.
Lozupone C, Hamady M, Knight R . UniFrac—an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics 2006; 7: 371.
Brawner KM, Morrow CD, Smith PD . Gastric microbiome and gastric cancer. Cancer J 2014; 20: 211–216.
Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 2009; 106: 2365–2370.
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE et al. A core gut microbiome in obese and lean twins. Nature 2009; 457: 480–484.
Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188.
Tremaroli V, Karlsson F, Werling M, Stahlman M, Kovatcheva-Datchary P, Olbers T et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab 2015; 22: 228–238.
Hartstra AV, Bouter KE, Backhed F, Nieuwdorp M . Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 2015; 38: 159–165.
Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N et al. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech 2013; 6: 443–456.
Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity 2012; 20: 738–747.
Brown K, DeCoffe D, Molcan E, Gibson DL . Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012; 4: 1095–1119.
Nagao-Kitamoto H, Kitamoto S, Kuffa P, Kamada N . Pathogenic role of the gut microbiota in gastrointestinal diseases. Intest Res 2016; 14: 127–138.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 2013; 110: 9066–9071.
Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016; 65: 426–436.
Ganesh BP, Klopfleisch R, Loh G, Blaut M . Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One 2013; 8: e74963.
Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med 2013; 11: 46.
Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007; 50: 2374–2383.
Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008; 455: 1109–1113.
Parks BW, Nam E, Org E, Kostem E, Norheim F, Hui ST et al. Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab 2013; 17: 141–152.
Zuo HJ, Xie ZM, Zhang WW, Li YR, Wang W, Ding XB et al. Gut bacteria alteration in obese people and its relationship with gene polymorphism. World J Gastroenterol 2011; 17: 1076–1081.
Guo Y, Liu CQ, Shan CX, Chen Y, Li HH, Huang ZP et al. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev 2016. e-pub ahead of print 30 August 2016; doi:10.1002/dmrr.2857.
Hatheway CL . Toxigenic clostridia. Clin Microbiol Rev 1990; 3: 66–98.
Cox LM, Blaser MJ . Antibiotics in early life and obesity. Nat Rev Endocrinol 2015; 11: 182–190.
Sabate JM, Coupaye M, Ledoux S, Castel B, Msika S, Coffin B et al. Consequences of Small Intestinal Bacterial Overgrowth in Obese Patients Before and After Bariatric Surgery. Obes Surg 2016. e-pub ahead of print 30 August 2016.
Acknowledgements
We thank Peter Eipers for sample processing and 16S analysis. The project described was supported by Award Number 5K01DK098319 (KMH) from the National Institute Of Diabetes And Digestive And Kidney Diseases. The following are acknowledged for their support of the Microbiome Resource at the University of Alabama at Birmingham: School of Medicine, Comprehensive Cancer Center (P30AR050948), Center for AIDS Research (5P30AI027767), Center for Clinical Translational Science (UL1TR000165) and Heflin Center.
Author contributions
TK and KMH were responsible for study conception and design, data analyses and interpretation, and drafting the article; TK and CLH generated experimental data; TP and CDM advised study concept, data analyses, and critical revision of the article. KMH is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Kim, T., Holleman, C., Ptacek, T. et al. Duodenal endoluminal barrier sleeve alters gut microbiota of ZDF rats. Int J Obes 41, 381–389 (2017). https://doi.org/10.1038/ijo.2016.224
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.224
This article is cited by
-
Changes in Gut Microbiota and Hormones After Bariatric Surgery: a Bench-to-Bedside Review
Obesity Surgery (2019)