Abstract
Objective:
To investigate whether the Cdc2-like kinase 2 (CLK2) is expressed in hypothalamic neurons and if it is, whether the hypothalamic CLK2 has a role in the regulation of energy balance.
Subjects:
Swiss mice on chow or high-fat diet (HFD) and db/db mice on chow diet were used to address the role of CLK2 in the hypothalamus.
Results:
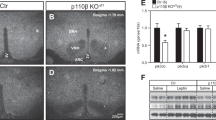
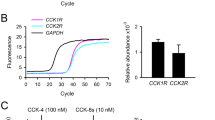
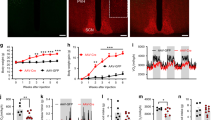
Hypothalamic CLK2Thr343 phosphorylation, which induces CLK2 activity, is regulated in vivo by refeeding, insulin and leptin, in a PI3K (phosphoinositide 3-kinase)-dependent manner. The reduction of CLK2 expression in the hypothalamus, by chronic pharmacological inhibition with TG003 or by chronic knockdown with small interfering RNA was sufficient to abolish the anorexigenic effect of insulin and leptin, to increase body weight, fat mass, food intake and to decrease energy expenditure in mice on chow. In contrast, CLK2Thr343 phosphorylation in the hypothalamus in response to insulin, leptin or refeeding was impaired in mice on HFD or in db/db mice. Chronic CLK2 inhibition in the hypothalamus was associated with a slight increase in the fasting blood glucose levels, reduction in PEPCK (phosphoenolpyruvate carboxykinase) expression in the liver and enhanced glucose production from pyruvate, suggesting a regulation of hepatic glucose production. Further, overexpressing CLK2 in the mediobasal hypothalami of mice on HFD or in db/db mice by adenovirus partially reversed the obese phenotype.
Conclusions:
Thus, our results suggest that protein CLK2 integrates some important hypothalamic pathways, and may be a promising molecule for new therapeutic approaches for obesity and diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Belgardt BF, Bruning JC . CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 2010; 1212: 97–113.
Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG et al. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes 2003; 52: 227–231.
Pardini AW, Nguyen HT, Figlewicz DP, Baskin DG, Williams DL, Kim F et al. Distribution of insulin receptor substrate-2 in brain areas involved in energy homeostasis. Brain Res 2006; 1112: 169–178.
Morton GJ, Schwartz MW . The NPY/AgRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 2001; 25: 56–62.
Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L et al. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci 2008; 28: 13640–13648.
Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ et al. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 2002; 22: 9048–9052.
Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab 2008; 7: 291–301.
Belgardt BF, Brüning JC . CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci 2010; 1212: 97–113.
Flak JN, Myers MG . Minireview: CNS mechanisms of leptin action. Mol Endocrinol 2016; 30: 3–12.
Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araújo EP, Velloso LA et al. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res 2005; 13: 48–57.
Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Woods SC, Schwartz MW, Baskin DG, Seeley RJ . Food intake and the regulation of body weight. Annu Rev Psychol 2000; 51: 255–277.
Woods SC, Seeley RJ . Adiposity signals and the control of energy homeostasis. Nutrition 2000; 16: 894–902.
Wong R, Balachandran A, Mao AY, Dobson W, Gray-Owen S, Cochrane A . Differential effect of CLK SR Kinases on HIV-1 gene expression: potential novel targets for therapy. Retrovirology 2011; 8: 47.
Yoshida T, Kim JH, Carver K, Su Y, Weremowicz S, Mulvey L et al. CLK2 is an oncogenic kinase and splicing regulator in breast cancer. Cancer Res 2015; 75: 1516–1526.
Rodgers JT, Haas W, Gygi SP, Puigserver P . Cdc2-like kinase 2 is an insulin-regulated suppressor of hepatic gluconeogenesis. Cell Metab 2010; 11: 23–34.
Tabata M, Rodgers JT, Hall JA, Lee Y, Jedrychowski MP, Gygi SP et al. Cdc2-like kinase 2 suppresses hepatic fatty acid oxidation and ketogenesis through disruption of the PGC-1α and MED1 complex. Diabetes 2014; 63: 1519–1532.
Nam SY, Seo HH, Park HS, An S, Kim JY, Yang KH et al. Phosphorylation of CLK2 at serine 34 and threonine 127 by AKT controls cell survival after ionizing radiation. J Biol Chem 2010; 285: 31157–31163.
Prada PO, Hirabara SM, de Souza CT, Schenka AA, Zecchin HG, Vassallo J et al. L-glutamine supplementation induces insulin resistance in adipose tissue and improves insulin signalling in liver and muscle of rats with diet-induced obesity. Diabetologia 2007; 50: 1949–1959.
Prada PO, Ropelle ER, Mourão RH, de Souza CT, Pauli JR, Cintra DE et al. EGFR tyrosine kinase inhibitor (PD153035) improves glucose tolerance and insulin action in high-fat diet-fed mice. Diabetes 2009; 58: 2910–2919.
Prada PO, Quaresma PG, Caricilli AM, Santos AC, Guadagnini D, Morari J et al. Tub has a key role in insulin and leptin signaling and action in vivo in hypothalamic nuclei. Diabetes 2013; 62: 137–148.
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004; 428: 569–574.
Quaresma PG, Reencober N, Zanotto TM, Santos AC, Weissmann L, de Matos AH et al. Pioglitazone treatment increases food intake and decreases energy expenditure partially via hypothalamic adiponectin/adipoR1/AMPK pathway. Int J Obes 2016; 40: 138–146.
Weissmann L, Quaresma PG, Santos AC, de Matos AH, Pascoal VD, Zanotto TM et al. IKKɛ is key to induction of insulin resistance in the hypothalamus, and its inhibition reverses obesity. Diabetes 2014; 63: 3334–3345.
Muotri AR, Marchetto MC, Zerbini LF, Libermann TA, Ventura AM, Sarasin A et al. Complementation of the DNA repair deficiency in human xeroderma pigmentosum group a and C cells by recombinant adenovirus-mediated gene transfer. Hum Gene Ther 2002; 13: 1833–1844.
Bidinosti M, Botta P, Kruttner S, Proenca CC, Stoehr N, Bernhard M et al. CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science 2016; 351: 1199–1203.
Vogt MC, Brüning JC . CNS insulin signaling in the control of energy homeostasis and glucose metabolism - from embryo to old age. Trends Endocrinol Metab 2013; 24: 76–84.
Muraki M, Ohkawara B, Hosoya T, Onogi H, Koizumi J, Koizumi T et al. Manipulation of alternative splicing by a newly developed inhibitor of Clks. J Biol Chem 2004; 279: 24246–24254.
Gautron L, Elmquist JK . Sixteen years and counting: an update on leptin in energy balance. J Clin Invest 2011; 121: 2087–2093.
Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996; 84: 491–495.
Obici S, Zhang BB, Karkanias G, Rossetti L . Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 2002; 8: 1376–1382.
Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007; 5: 438–449.
Roh E, Song do K, Kim MS . Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp Mol Med 2016; 48: e216.
Rojas JM, Schwartz MW . Control of hepatic glucose metabolism by islet and brain. Diabetes Obes Metab 2014; 16: 33–40.
Inoue H, Ogawa W, Asakawa A, Okamoto Y, Nishizawa A, Matsumoto M et al. Role of hepatic STAT3 in brain-insulin action on hepatic glucose production. Cell Metab 2006; 3: 267–275.
Acknowledgements
We are grateful for financial support from State University of Campinas (FUNCAMP), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo): Auxílio Regular 2012/10338-6 and 2015/00343-0 and CEPID 2013/07607-8, São Paulo, Brazil and from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) Universal (481084/2013-4) and INCT (Instituto Nacional Ciência e Tecnologia de Obesidade e Diabetes; 573856/2008-7). The authors acknowledge the technical assistance of Luis Janeri, Jósimo Pinheiro, Dioze Guadagnini, Lígia Parreira Muniz and Ramon Henrick Zorzeto dos Santos from the Department of Internal Medicine, State University of Campinas. We thank Professor Armando Morais Ventura for kindly provide pShires vector. The present work received financial support from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo): Auxílio Regular 2012/10338-6 and CEPID 2013/07607-8, São Paulo, Brazil and from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) Universal (481084/2013-4) and INCT (Instituto Nacional Ciência e Tecnologia de Obesidade e Diabetes) (573856/2008-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
PGFQ designed, planned and performed all the experiments, analyzed data and wrote the manuscript; TMZ, ACS and LW performed experiments; JDJ and ICF performed and analyzed the immunohistochemistry assays; AHBM and ILC contributed with siRNA assays designing; FMS and PGFQ performed the adenovirus production and purification and reviewed the manuscript; POP directed the study, provided financial support and reviewed data analysis and the manuscript.
Rights and permissions
About this article
Cite this article
Quaresma, P., Weissmann, L., Zanotto, T. et al. Cdc2-like kinase 2 in the hypothalamus is necessary to maintain energy homeostasis. Int J Obes 41, 268–278 (2017). https://doi.org/10.1038/ijo.2016.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.174