Abstract
Background:
Biotin acts as a coenzyme for carboxylases regulating lipid and amino-acid metabolism. We investigated alterations of the biotin-dependent functions in obesity and the downstream effects of biotin restriction in adipocytes in vitro.
Subjects:
Twenty-four monozygotic twin pairs discordant for body mass index (BMI). Mean within-pair difference (heavy-lean co-twin, Δ) of BMI was 6.0 kg m–2 (range 3.1–15.2 kg m–2).
Methods:
Adipose tissue (AT) DNA methylation, gene expression of AT and adipocytes, and leukocytes (real-time quantitative PCR), serum biotin, C-reactive protein (CRP) and triglycerides were measured in the twins. Human adipocytes were cultured in low and control biotin concentrations and analyzed for lipid droplet content, mitochondrial morphology and mitochondrial respiration.
Results:
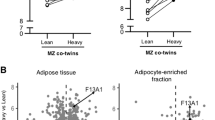
The gene expression levels of carboxylases, PCCB and MCCC1, were upregulated in the heavier co-twins’ leukocytes. ΔPCCB (r=0.91, P=0.0046) and ΔMCCC1 (r=0.79, P=0.036) correlated with ΔCRP within-pairs. Serum biotin levels were lower in the heavier (274 ng l–1) than in the lean co-twins (390 ng l–1, P=0.034). ΔBiotin correlated negatively with Δtriglycerides (r=–0.56, P=0.045) within-pairs. In AT, HLCS and ACACB were hypermethylated and biotin cycle genes HLCS and BTD were downregulated (P<0.05). Biotin-dependent carboxylases were downregulated (ACACA, ACACB, PCCB, MCCC2 and PC; P<0.05) in both AT and adipocytes of the heavier co-twins. Adipocytes cultured in low biotin had decreased lipid accumulation, altered mitochondrial morphology and deficient mitochondrial respiration.
Conclusions:
Biotin-dependent functions are modified by adiposity independent of genetic effects, and correlate with inflammation and hypertriglyceridemia. Biotin restriction decreases lipid accumulation and respiration, and alters mitochondrial morphology in adipocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLOS Med 2008; 5: e51.
Pietiläinen KH, Rog T, Seppänen-Laakso Virtue S, Gopalacharyulu P, Tang J et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLOS Biol 2011; 9: e1000623.
Nunnari J, Suomalainen A . Mitochondria: in sickness and in health. Cell 2012; 148: 1145–1159.
McGarry JD . Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002; 51: 7–18.
Muoio DM . Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 2014; 159: 1253–1262.
Malandrion MI, Fucho R, Weber M, Calderon-Dominguez M, Mir JF, Valcarcel L et al. Enhanced fatty acid oxidation in adipocytes and macrophages reduces lipid-induced triglyceride accumulation and inflammation. Am J Physiol Endocrinol Metab. 2015; 308: 756–769.
Hymes J, Fleischhauer K, Wolf B . Biotinylation of histones by human serum biotinidase: assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency. Biochem Mol Med 1995; 56: 76–83.
Pestinger V, Wijeratne SS, Rodriguez-Melendez R, Zempleni J . Novel histone biotinylation marks are enriched in repeat regions and participate in repression of transcriptionally competent genes. J Nutr Biochem 2011; 22: 328–333.
Maebashi M, Makino Y, Ohinata K, Kimura S, Takao S . Therapeutic evaluation of the effect of biotin on hyperglycemia in patients with non-insulin diabetes mellitus. J Clin Biochem Nutr 1993; 14: 211–218.
Marshall MW, Kliman PG, Washington VA, Mackin JF, Weinland BT . Effects of biotin on lipids and other constituents of plasma of healthy men and women. Artery 1980; 7: 330–351.
Larrieta E, Velasco F, Vital P, López-Aceves T, Lazo-de-la-Vega-Monroy ML, Rojas A et al. Pharmacological concentrations of biotin reduce serum triglycerides and the expression of lipogenic genes. Eur J Pharmacol 2010; 644: 263–268.
Báez-Saldaña A, Zendejas-Ruiz I, Revilla-Monsalve C, Islas-Andrade S, Cárdenas A, Rojas-Ochoa A et al. Effects of biotin on pyruvate carboxylase, acetyl-CoA carboxylase, propionyl-CoA carboxylase, and markers for glucose and lipid homeostasis in type 2 diabetic patients and nondiabetic subjects. Am J Clin Nutr 2004; 79: 238–243.
Ollikainen M, Ismail K, Gervin K, Kyllönen A, Hakkarainen A, Lundbom J et al. Genome-wide blood DNA methylation alterations at regulatory elements and heterochromatic regions in monozygotic twins discordant for obesity and liver fat. Clin Epigenetics 2015; 7: 39–51.
Heinonen S, Buzkova J, Muniandy M, Kaksonen R, Ollikainen M, Ismail K et al. Impaire mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes 2015; 64: 3135–3145.
Kaprio J, Pulkkinen L, Rose RJ . Genetic and environmental factors in health-related behaviors: studies on Finnish twins and twin families. Twin Res 2002; 5: 366–371.
Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S . A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013; 29: 189–196.
Leek JT, Storey JD . Capturing heterogeneity in gene expression studies by surrogate variable analysis. PLOS Genet 2007; 3: 1724–1735.
Wu Z, Irizarry RA, Gentleman R, Murillo FM, Spencer F . A model based background adjustment for oligonucleotide expression arrays. J Am Stat Association 2004; 99: 909–917.
Van Harmelen V, Skurk T, Hauner H . Primary culture and differentiation of human adipocyte precursor cells. Methods Mol Med 2005; 107: 125–135.
Skurk T, Hauner H . Primary culture of human adipocyte precursor cells: expansion and differentiation. Methods Mol Biol 2012; 806: 215–226.
Smyth GK, Michaud J, Scott HS . Use of within-array replicate spots for assessing differential expressions in microarray experiments. Bioinformatics 2005; 21: 2067–2075.
Naukkarinen J, Heinonen S, Hakkarainen A, Lundbom J, Vuolteenaho K, Saarinen L et al. Characterising metabolically healthy obesity in weight-discordant monozygotic twins. Diabetologia 2014; 57: 167–176.
Heinonen S, Saarinen L, Naukkarinen J, Rodríguez A, Frühbeck G, Hakkarainen A et al. Adipocyte morphology and implications for metabolic derangements in acquired obesity. Int J Obes 2014; 38: 1423–1431.
Abu-Elheiga L, Wu H, Gu Z, Bressler R, Wakil SJ . Acetyl-CoA carboxylase 2-/- mutant mice are protected against fatty liver under high-fat, high-carbohydrate dietary and de novo lipogenic conditions. J Biol Chem 2012; 287: 12578–12588.
Olson DP, Pulinilkunnil T, Cline GW, Shulman GI, Lowell BB . Gene knockout of Acc2 has little effect on body weight, fat mass, or food intake. Proc Natl Acad Sci USA 2010; 107: 7598–7603.
Kuri-Harcuch W, Wise LS, Green H . Interruption of the adipose conversion of 3T3 cells by biotin deficiency: differentiation without triglyceride accumulation. Cell 1978; 14: 53–59.
Gomes LC, Di Benedetto G, Scorrano L . During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 2011; 13: 589–598.
Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J . Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA 2011; 108: 10190–10195.
Rambold AS, Cohen S, Lippincott-Schwartz J . Fatty acid trafficking in starved cells: regulation by lipid droplet lipolysis, autophagy, and mitochondrial fusion dynamics. Dev Cell 2015; 32: 678–692.
Virtue S, Vidal-Puig A . Adipose tissue expandability, lipotoxicity and the metabolic syndrome – an allostatic perspective. Biochim Biophys Acta 2010; 1801: 338–349.
Abu-Elheiga L, Oh W, Kordari P, Wakil SJ . Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci USA 2003; 100: 10207–10212.
Tong L . Structure and function of biotin-dependent carboxylases. Cell Mol Life Sci 2013; 70: 863–891.
Pacheco-Alvarez D, Solórzano-Vargas RS, Del Río AL . Biotin in metabolism and its relationship to human disease. Arch Med Res 2002; 33: 439–447.
Pérez-Monjaras A, Cervantes-Roldán R, Meneses-Morales I, Gravel RA, Reyes-Carmona S, Solórzano-Vargas S et al. Impaired biotinidase activity disrupts holocarboxylase synthetase expression in late onset multiple carboxylase deficiency. J Biol Chem 2008; 283: 34150–34158.
Acknowledgements
We want to thank all the volunteers for their valuable contribution, staff members of the Obesity Research Unit, especially Saila Saarinen, Mia Urjansson, Katja Sohlo and Miia Juntunen and Anna-Maija Honkala for technical assistance, Mervi Lindman from the Electron microscopy unit at the Institute of Biotechnology and Jussi Kenkkilä from the Biomedicum Imaging Unit for technical expertise and Uwe Richter for scientific advise. This study was supported by Helsinki University Hospital Research Funds and grants from the Novo Nordisk Foundation (KP), Diabetes Research Foundation (KP, SH), Jalmari and Rauha Ahokas Foundation (KP, LB), Orion Pharmos Foundation (SH), Emil Aaltonen Foundation (SH), Finnish Medical Foundation (SH), Finnish Foundation for Cardiovascular Research (KP), Finnish Funding Agency for Innovation (SM), Sigrid Juselius Foundation (MO), University of Helsinki Funds 490139 (MO), and Academy of Finland (265240, 263278 (JK), 251316 (MO), EPITRAIN - FP7-PEOPLE-2012-ITN, grant agreement 316758 (JK, MO) and 266286 and 272376 (KP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Rights and permissions
About this article
Cite this article
Järvinen, E., Ismail, K., Muniandy, M. et al. Biotin-dependent functions in adiposity: a study of monozygotic twin pairs. Int J Obes 40, 788–795 (2016). https://doi.org/10.1038/ijo.2015.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.237
This article is cited by
-
Effects of biotin on promoting anammox bacterial activity
Scientific Reports (2021)
-
Epigenetic Effects of the 13 Vitamins
Current Pharmacology Reports (2018)